CAs expectations regarding the quality and precision of products continue to grow, further advances in materials engineering are strictly associated with the use of computer assisted computation methods. Computer modelling and simulations facilitate the design of engineering materials and predicting the properties of these materials with significant reduction in time and costs. Thus, the literature contains numerous reports on the subject (1-11). Computational support is particularly required in vacuum thermochemical processing due to its non-equilibrial nature and transient states in the course of the processes. In this case, the primary goal of simulation is to predict the course of the process and the final properties of the product, thus ensuring the repeatability of process outcomes. The vacuum carburizing model presented in this article is a classic example of a hybrid model, in which:
- basic phenomena of carburizing low- and medium-carbon steels are described by an analytical mathematical-physical model;
- parameters such as carbon diffusion coefficient DC and carbon transfer coefficient β were determined by analysis of variance and multiple regression on the basis of experimental data sets;
- phenomena that could not be described by mathematical equations or for which no mathematical apparatus was available were processed by means of data-mining using neural networks;
- phenomena of formation and dissolution of carbon deposits were described by a heuristic model;
- material data regarding steels were collected in a database to eliminate redundant processes and accelerate model operation.
A Mathematical-Physical Model of the Vacuum Carburizing Process
The mathematical-physical model of the vacuum carburizing process is based on six principles defined by the authors of references [12-14]:
- Carburizing atmosphere in the saturation stage is delivered to the sample surface in a continuous manner.
- Composition of carburizing atmosphere remains constant throughout the process.
- Carbon atoms are released as a result of catalytic interaction between the atmosphere and the carburized surface.
- Carbon is transported to the material by means of diffusion in accordance with Fick’s equations.
- Carbon diffusion coefficient in austenite depends on the temperature, carbon concentration and the presence of alloy additives.
- Computations are based on a semi-infinite area.
The development of carbon profiles in austenite depends both on the phenomena occurring at the gas-metal interface, and on the diffusion of carbon in austenite. Phenomena occurring at the gas-metal interface that determine the rate in which carbon concentration changes at the surface, including the most important catalytic interactions between the carburizing atmosphere, are reflected in the mathematical model by atomic carbon flux IC crossing the interface:
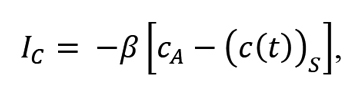
where: IC – flux of carbon atoms across the surface of carburized material, cA – concentration of carbon at equilibrium with the carburizing atmosphere,( c( t))s– current concentration of carbon at the material surface, β – coefficient of carbon transport across the gas-metal interface. Carbon transport in austenite has been described on the basis of Fick’s diffusion laws. The first law described the flux of the diffusing component:
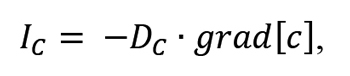
where: Dc – carbon diffusion coefficient in austenite; the value depends on the temperature, carbon concentration, and the content of alloy additives, grad[c]– gradient of carbon concentration in austenite.
The second law describes the change in carbon concentration as a function of time:
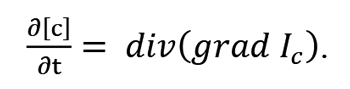
In the case of single-directional diffusion the above laws can be expressed in the following manner:
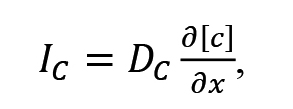
The effect of surface phenomena on the profile of carbon diffusion within austenite is taken into consideration by solving Fick’s second law at the border condition resulting from the comparison of equations (1) and (4):
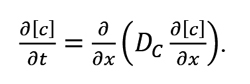
Therefore, the problem of determining the profile of carbon concentrations within austenite amounts to solving equation (5) at the border condition (6) and the initial condition (7):
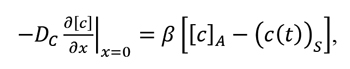
Computational Model of the Vacuum Carburizing Process
Input computational data included process parameters, chemical composition of the material, carbon carrier gas parameters and the geometry of the carburized surface. Coefficients affecting model precision are carbon diffusion coefficient DC, activity of carbon in austenite aC, carbon transfer coefficient β and geometry coefficient.
Functions describing the relationship between the carbon diffusion coefficient DC and temperature or time were presented in numerous articles [15-24]. The first studies of the model were based on the carbon diffusion coefficient formula proposed by Goldstein [15]. This relationship soon turned out to be insufficient, and therefore formulas proposed by Leyens [16], Tibbets [17] and the empirical equation proposed by Dybowski in an article dedicated to this issue were successively used in subsequent studies [25]. The last equation provided for the best agreement of the model with actual profiles within the carburizing process temperatures of 860- 1010°C.
One of the factors impacting the value of carbon diffusion coefficient DC is the activity of carbon within austenite in the presence of alloy additives aC, with the effect of alloy additives on carbon concentration profile being taken into consideration. In addition, the coefficient defining the effect of alloy additives on the activity of carbon within austenite aC allows for the computation of the maximum solubility of carbon within austenite, Cgr, at temperature T for a particular material.Therefore, aC value was included in the model, making use of the equation provided in [20].
Experimental data set results were used to determine the carbon transfer coefficient β. Multiple regression analysis revealed that the only factor having a statistically significant impact on the value of the carbon transport coefficient is temperature, related to the coefficient by equation (8):
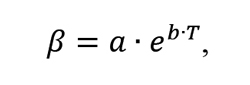
where: β – carbon transfer coefficient, T – temperature, a,b – coefficients.
For better precision of calculations the computational model accounted for the effect of surface geometry on the distribution of carbon within the carburized layer by introducing a correction factor μi based on the data presented in [13]:
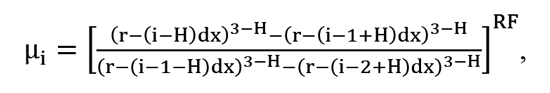
[9]
where: i – spatial sample of interest in the Crank-Nicolson method, r – curvature radius, H, RF – coefficients listed in Table 1.
Computation Algorithm
The first mathematical algorithm for vacuum carburizing was by Kołodziejczyk in an article dedicated to this topic [13]. Model input parameters include the process, material and surface geometry parameters. The material status is calculated in a matrix consisting of cells representing spots located within the material at every 0.01 mm, in time increments of 0.1 s. Limitations expressed by condition (10) were set onto the time increment and computational step to ensure the convergence and stability of computations. The stability of partial differential computations means that errors are not accumulated as the problem is being solved, while convergence of computations means that an accurate result is obtained in the solution.
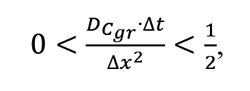
[10]
where: DCgr– carbon diffusion coefficient calculated for the maximum concentration of carbon within austenite, Dt– time increment, Dx– computational step.
Calculations start at the time point t=0 and are continued until the process time TPROC reaches the total time resulting from the total of segments (steps) defined in input parameters. The value of the diffusion coefficient during the process depends on several factors simultaneously; thus, the equation describing unidimensional transport of carbon within austenite becomes non-linear and impossible to solve analytically. Therefore, the issue is solved by numerically using the Crank- Nicolson finite-differences method [26], as well as the Crout factorization and Thomas algorithm.
Modelling of Formation and Dissolution of Carbide Precipitations
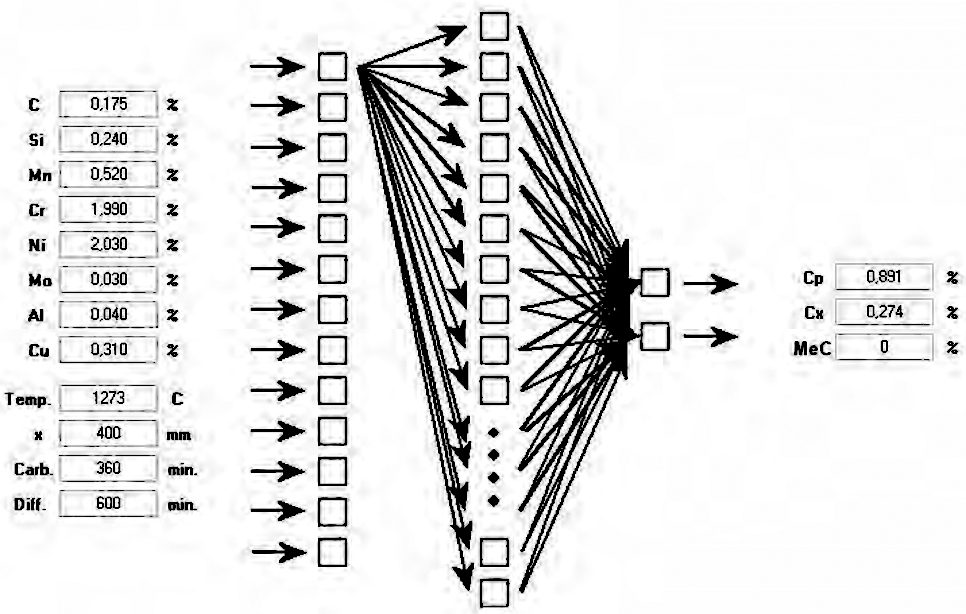
Carburizing processes carried out above the limit concentration Cgr lead to carbide precipitations. These precipitations are gradually dissolved in the hold stage; however, the kinetics of both formation and dissolution follow individual patterns, strongly related to the chemical composition of a particular precipitation. Since the literature lacks comprehensive information on the kinetics of the process, the black box data-mining model was used, as it required no analytical knowledge of the phenomenon of interest. A series of experimental studies were also conducted in order to better understand this issue; the results are presented in publications [27, 28, 29]. A learning data set was developed on the basis of these results, followed by the development of a neural network reflecting the relationships between process parameters and the properties of the carburized layer, as was also described in detail in the above papers. When choosing the network architecture it was assumed that the network should be extrapolative, i.e. it should possibly correctly predict cases beyond the learning set. This assumption prevailed in the choice of the Multi Layer Perceptron (MLP) networks due to their capabilities in this respect [30].
Carburizing process parameters were defined as network input parameters, including carburizing temperature TN, segmentation and chemical composition of the material: concentrations of carbon C, silicone Si, manganese Mn, chromium Cr, nickel Ni, aluminum Al, copper Cu; as well as the point of consideration X within the material. The output network signals represented the properties of the carburized layer: surface carbon concentration, CP; carbon concentration at distance X from the surface, CX; as well as the percentage content of carbides at distance X from the surface, MeC (Figure 1).
Conclusions
From the industrial standpoint, the quality and usefulness of a technology depends on its ability to ensure required product parameters and repeatability of the process. Therefore, simulation programs based on mathematical models and artificial intelligence methods provide much-required support for the technical features of modern vacuum furnaces, serving as tools for the design and optimization of these furnaces, thus ensuring optimum control of the production process.
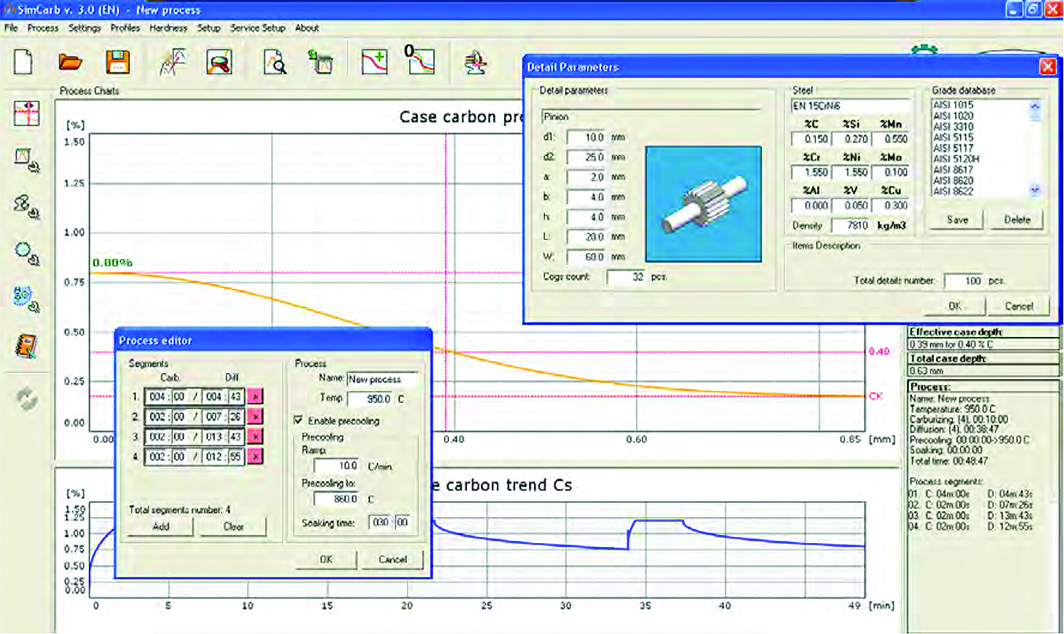
The FineCarb® vacuum carburizing technology, followed by gas or oil hardening, is used, e.g. in the production of drive and motion-transferring elements in engineering, in the automotive and aviation industries for the hardening of outer layers of gears, shafts, bearing rings, levers, etc. The presented vacuum carburizing models, along with the gas hardening model, were included in the expert system (Figure 2) that supports the technology.
Acknowledgments
The publication was financed as part of the project No. UODDEM- 1-193/001. The project is conducted in the framework of a pilot venture “Support research and development on a demonstration scale”.
References
- Liujie, X. et al., “Artificial neural network prediction of heat-treatment hardness and abrasive wear resistance of high-vanadium high-speed steel (HVHSS)”, Journal of Materials Science, Vol. 42 (2007), pp. 2565-2573.
- Liujie, X., et al., “Artificial neural network prediction of retained austenite content and impact toughness of highvanadium high-speed steel (HVHSS)”. Materials Science and Engineering A, Vol. 433 (2006), pp. 251-256.
- Jacquet, P. et al., “A novel technique to monitor carburizing processes”, Materials Chemistry and Physics, Vol. 77 (2002), pp. 542-551.
- Larsson, H. et al., “Gas nitriding of high vanadium steels – experiments and simulations”, Metallurgical and Materials Transactions A, Vol. 35 (2004), pp. 2799-2802.
- Malinova, T. et al., “Simulation of microhardness profiles for nitrocarburized surface layers by artificial neural network”, Surface and Coatings and Technology, Vol. 135 (2001), pp. 258-267.
- Smoljan, et al., “Prediction of mechanical properties of quenched and tempered steel die”, 3rd Int’l Conference on Heat Treatment and Surface Engineering of Tools and Dies, Wels, March 2011.
- Sommer: “Process audits in heat treatment shops as possibilities of a systematic process improvement”, 3rd Int’l Conference on Heat Treatment 2011 and Quality in Heat Treatment, Wels, March 2011.
- Wołowiec, Kula, et al., “Simulation and control of tool steel quenching process”, 25th European Conference on Modelling and Simulation, Kraków, June 2011, pp. 357- 361.
- Dobrzański, L.A. et al., “Computer aided method for evaluation of failure class of materials working in creep conditions”, Journal of Materials Processing Technology, Vol. 157-158 (2004), pp. 102-106.
- Wołowiec, E. et al., “Computer tools to support thermochemical treatment”, Industrial Furnaces & Boilers, Vol. 11-12 (2011), pp. 8-14 (in Polish).
- Wołowiec, E. and Kula, P., “Practical application of artificial neural networks in designing parameters of steel heat treatment processes”, Lecture Notes of Computer Science, Vol. 7267 (2012), pp. 196-203.
- Kula, et al., “Computer-aided process vacuum carburizing”, 4th Seco/Warwick Group Seminar, Lagow, September 2002, pp. 1-14.
- Kołodziejczyk, Ł., Mathematical modeling of the process of vacuum carburizing, Technical University of Lodz (Lodz, 2003).
- Kula, P. et al., “The application of numerical model in designing of vacuum carburizing”, Materials Engineering, Vol. 4 (2001), pp. 511-513 (in Polish).
- Goldstein, J.L. and Moren, A.E., “Diffusion modeling of the carburization process”, Trans. of AIME, Vol. 9A (1978).
- Leyens, G. et al., “Berechnung der Aufkohlung nach dem Saettigungs-Ausgleichs-Verfahren”, Arch. Eisenhuttenwes, Vol 47, No. 6 (1976), pp. 385-390.
- Tibbets, G., “Diffusity of carbon in iron and steels at high temperatures”, Journal of Applied Physics, Vol. 51 (1980), pp. 4813-4816.
- Argen, J., “A revised expression for the diffusity of carbon in binary Fe-C austenite”, Scripta Metallurgica, Vol. 20 (1986), pp. 1507-1510.
- Bhandesia, H.K., “Diffusion of carbon in austenite”, Metal Science, Vol. 15 (1981), pp. 477-479.
- AWT-Fachausschuss, Die prozessregelung beim Gasaufkohlen und Einsatzharten, Expert Verlag (Renningen-Malmsheim, 1997).
- Coates, D.E., “Diffusional growth limitation and hardenability”, Metallurgical Transactions, Vol. 4 (1973), pp. 2313-2325.
- Collin, R. et al., “A mathematical model for predicting carbon concentration profiles of gas-carburized steel”, J. Iron Steel Inst., Vol. 10 (1972), pp. 785-789.
- Hirschheimer, “The mathematical basis for carburizing process variables on vacuum carburizing”, 2nd Int. Conference on Carburizing and Nitriding with Atmospheres, Cleveland, 1995, pp. 103-108.
- Wells, A. and Batz, W., “Diffusion coefficient of carbon in austenite”, Trans. AIME, Vol. 188 (1950), pp. 553-560.
- Dybowski, K., The computing of effective carbon diffusion coefficient in steels to a process of vacuum carburizing control, Technical University of Lodz (Lodz, 2005).
- Crank, J., The mathematics of diffusion, Oxford University Press (New York, 1979).
- Wołowiec, E. and Kula, P., “The application of artificial neural networks in designing single-segment processes of vacuum carburizing” Materials Engineering, Vol. 3 (2010), pp. 712-715.
- Wołowiec, E., The application of artificial intelligence methods in development and technical realization of surface engineering processes, Technical University of Lodz (Lodz, 2009).
- Wołowiec, E. and Kula, P., “The application of artificial neural networks in optimization of heat treatment processes of steel”, Journal of Applied Computer Science, Vol. 19, No. 1 (2011), pp. 161-169.
- Hagan, M. et al., Neural Networks Design, PWS Publishing Company