Natural gas is a clean-burning fossil fuel that, when ignited with air, produces heat, carbon dioxide (CO2), and water in vapor form (H2O). Mix one cubic foot of gas plus 10 cubic feet of air, a spark and the result will be one cubic foot of CO2 plus two cubic feet of H2O and eight cubic feet of nitrogen. Two cubic feet of water vapor equals 3.2 tablespoons of water.
Nitrogen obviously does not burn, but it still must be heated and contributes to the convection heat transfer of energy.
Anyone who has used a ventless natural gas room space heater can attest to the quantity of water that condenses on windows in cold climates. A 20,000 BTU heater requires 200 cubic feet of natural gas plus 2,000 cubic feet of room air. Per the formula above, the resulting combustion will produce 200 cubic feet of CO2 plus 400 cubic feet of H2O, or about two quarts per hour.
Gas-fired heat-treating furnaces use natural gas in two ways: They heat the process hot zone with a stoichiometric mixture (ideal combustion) of one part of natural gas to 10 parts of air to produce the endothermic atmosphere for carburizing.
Since no combustion reaction is perfect, it’s possible that a small fraction or parts per million (ppm) of CO could be produced, therefore excess air is always used. Heat-treating furnaces, due to their operation in large industrial plants, release effluents into a hood mounted to the plant roof most of the time. It’s not unusual to see some plants allowing the burner effluents to vent directly into the open area up to the plant ceiling where, typically, exhaust fans vent gases outside the plant. Where gases vent directly into the plant air, they mix with plant environment to form what we call an “indiscriminate atmosphere” — meaning that combustion products will be diluted in air.
Heat-treating furnaces can be gas heated with two methods: direct or indirect fired. Direct firing consists of burners placed in the refractory walls pointing just above and/or just below the parts being heated. Burners are never positioned to fire directly at parts since the temperature of the flame is near 2,000°F (1,093°C). Special flat-flame burners are the exception where the burner flame is designed to spread along the refractory wall, not straight out as a jet. Direct firing produces CO2, H2O, and nitrogen as mentioned above. Small amounts in the ppm range of oxides of nitrogen (NOx) are also produced. Since both CO2, and H2O contain oxygen, they will, depending on temperature, be oxidizing to iron and steel. Where oxidation cannot be tolerated, indirect firing is the option.
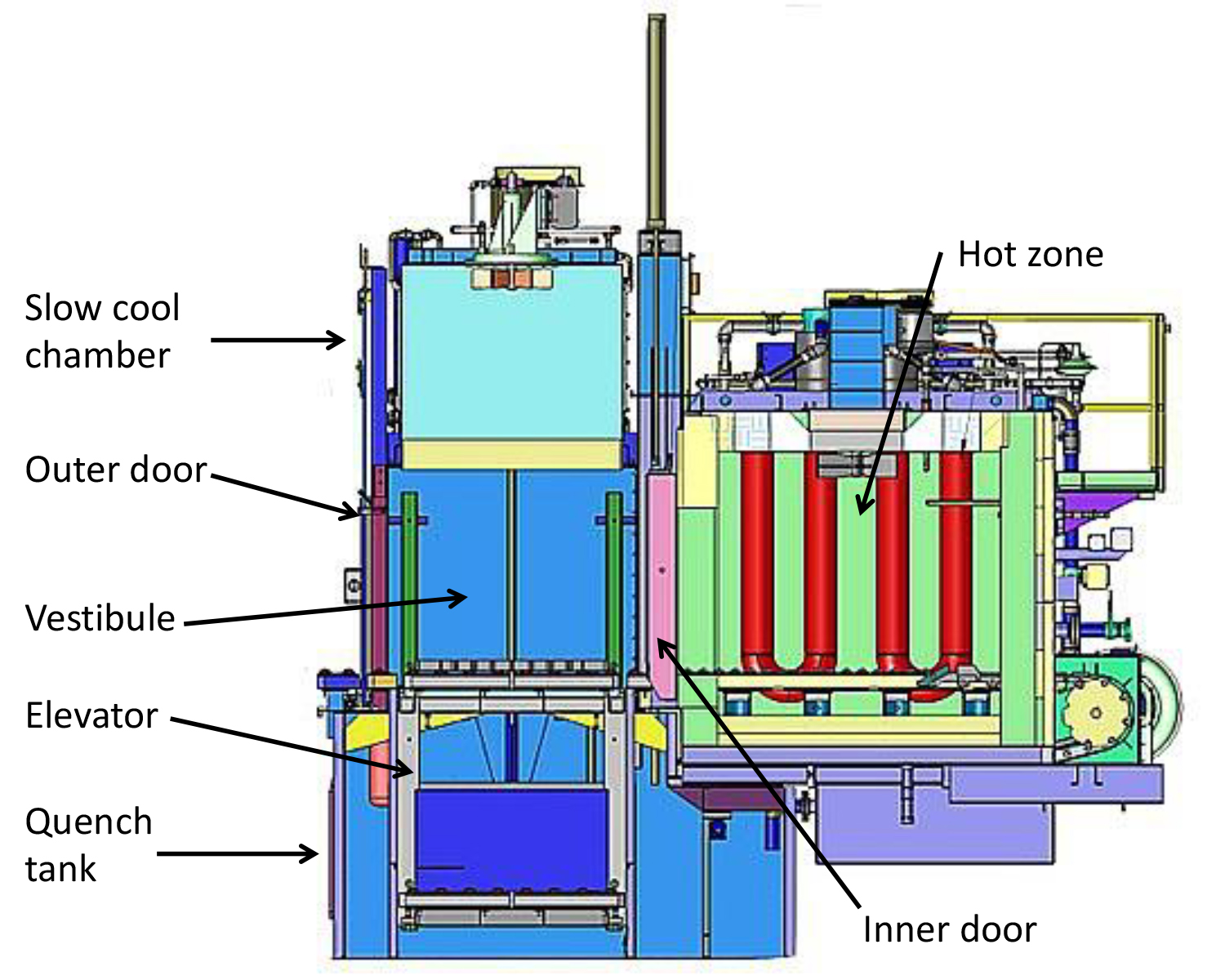
Indirect fired furnaces attach burners to radiant tubes made from heat-resisting nickel, chromium, and iron alloys. This method is used where a special atmosphere is used to protect parts with nitrogen or carburize with endothermic gas. Endo gas consists of 20 percent CO, 40 percent hydrogen, and 40 percent nitrogen with trace amounts for CO2, H20, and methane (CH4). We all know hydrogen, like natural gas, can be explosive if not handled properly. Heat-treating furnaces are designed to handle those gases appropriately. Take, for example, the batch furnace represented in my March/April Hot Seat column shown in Figure 1. In general, most people outside the heat-treating industry, and many even within the arena, believe that explosive gases and heat are not a good combination. Here’s the truth:
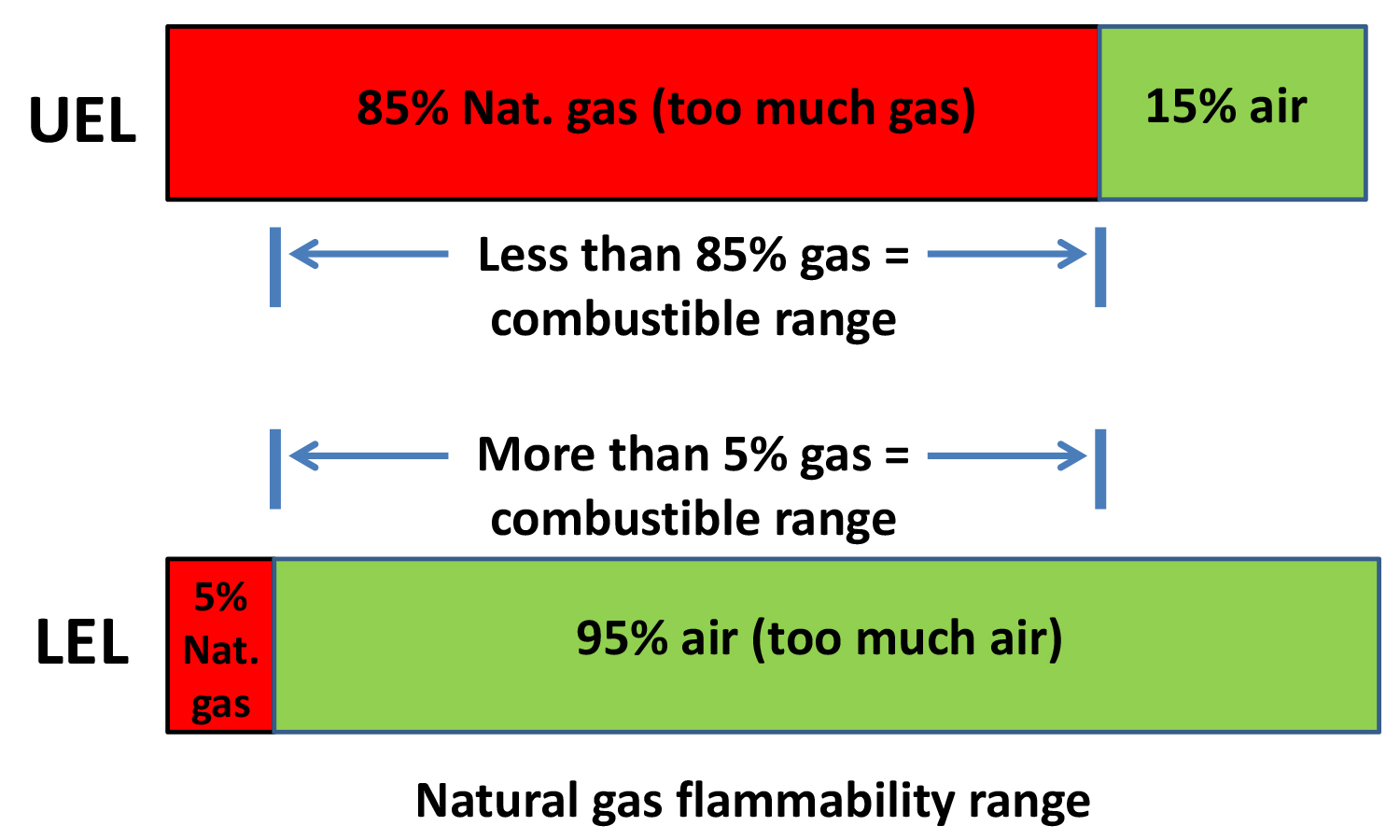
Whenever I encounter a furnace with a combustible atmosphere, I want it to be above 1,400°F (760°C). Why? 1,400°F is the auto ignition temperature above which natural gas and hydrogen will burn with air if they’re within the combustible limit. The situation you want to avoid is combustible gases mixed with air below the ignition temperature; this is how explosions occur with premix combustible gases. For fuels such as natural gas and hydrogen to burn, the ratios of air-to-fuel must fall between the lower explosive limit (LEL) and the upper explosive limit (UEL). Anywhere outside of those limits, combustion can’t occur. For natural gas, the LEL is 5 percent — meaning if the gas concentration in air is less than 5 percent, it’s too lean to burn or explode. If the gas concentration is high, allowing only 15 percent of air, it’s too rich to burn or explode. So, for an explosion to occur the mix must be between 5 to 15 percent air. Hydrogen’s LEL and UEL are 4 percent and 75 percent respectively. The other combustible gas in endo is CO. Its LEL is 12.5 percent and UEL is 74 percent. The limits can be explained further in FIgure 2.
Carburizing is the most employed heat-treating process because it has the greatest influence on ferrous alloy’s strength properties. Carbon is added to the steel’s surface by endothermic gas mentioned above. And as can be seen, it contains 40 percent hydrogen. So how can we as furnace designers and heat treaters safely use it? By controlling how and when it comes into contact with air.
Today, many heat treaters — commercial or in-house — have nitrogen stored as liquid and vaporized to gas. When endo gas is required, the furnace is first heated to above 1,400°F (760°C), then filled with five volume changes of nitrogen to displace the air. Five volume changes will reduce the oxygen to 0.67 percent of 21 percent or 1,415 ppm. Once five volume changes have occurred, endo gas is then added to purge the nitrogen. Nitrogen is a huge advantage in regions where electrical power is frequently lost because, when power drops, furnaces cool. And as stated, you don’t want a furnace with endo gas cooling below 1,400°F. In such an event, emergency nitrogen immediately flows into the furnace(s), displacing the endo gas. If nitrogen is available, the stored capacity must accommodate the volume of furnaces in the facility.

Before the widespread availability of nitrogen heat treats that could experience lengthy power outages, the only option was to burn out the endo gas before the hot zone cooled below 1,400°F. If many furnaces are involved, this could be a pretty daunting chore. Burning out a batch furnace without nitrogen involves the following:
- Manually open the outer, or vestibule, door(s) which will ignite the flame screen under the door opening. The flame screen shown in Figure 3 is used to fill the vestibule with flue gas and remove oxygen.
- While the flame screen is burning, open the inner door, and the residual endo gas in the hot zone (the endo gas inlet valve would have closed with loss of power) will begin to be consumed. The flame will appear to recede toward the rear and top of the hot zone as air from the vestibule supports its combustion.
- The flame screen can be shut off, and the remaining endo gas will continue to burn out in a few seconds.
- Obviously, any parts still in the hot zone will be oxidized and of no use.
- When adding endo gas to a hot furnace after an air burnout with nitrogen, proceed as follows:
- Always start by opening the vestibule or outer door, and turn the flame screen off.
- Introduce nitrogen for five volume changes.
- Turn off nitrogen and add endo gas.
- Place a natural gas torch or lance at the opening in the inner door, center or at the bottom.
- When endo gas begins to burn at the opening, remove the torch.
- Turn on the flame screen and allow the flame to burn into the vestibule for about five minutes to consume oxygen and fill the vestibule with flue gas, CO2, and water vapor.
- Depending on the volume of the vestibule, endo gas will begin to burn at the vestibule effluent in a few minutes, indicating that the entire furnace has been purged and is ready to use.
- When nitrogen is not available and with a furnace above 1,400°F filled with air, endo gas can be added per the following:
- Open the vestibule or outer door, and turn off the flame screen.
- Place a natural gas torch or lance at the opening in the inner door, center, or at the bottom.
- Turn on endo gas.
- As the endo gas enters the air-filled hot zone, a flame will appear at the point of gas entry.
- As the endo gas consumes oxygen in the hot zone, the oxygen percentage will continue to drop until the LEL for natural gas, CO, and hydrogen has been reached and the flame will disappear, but endo will continue to replace the remaining air.
- When the endo gas begins to burn out of the inner door vent, the natural gas torch can be removed and the flame screen turned on.
- The flame screen is allowed to fill the vestibule for a few minutes and the vestibule or outer door can be closed.
- A minute or so later, when the vestibule has been filled with endo gas, the vestibule effluent will begin to exhibit the flame.
- At this point, the entire system has been purged with endo gas, and the furnace can be used.
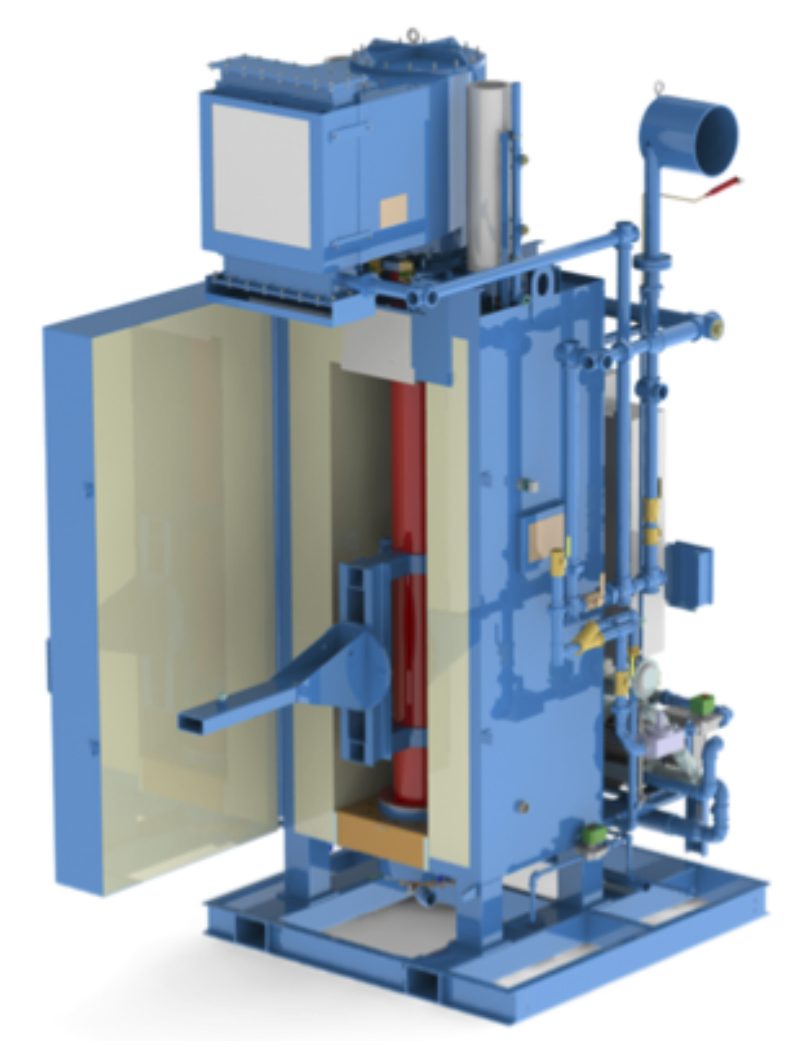
Endothermic generators like AFC-Holcroft’s EZ® endo generator, Figure 4, produce gas for carburizing by passing natural gas and air over a nickel catalyst at 1,950°F (1,065°C). More on how heat-treating furnaces use endo gas for carburizing in part 3.



























