Sustainability is a key value driver and focus for industrial manufacturing, and additive manufacturing fulfills that need in more ways than one.
Additive manufacturing, a rapidly maturing industrial technology, has great potential to reduce the energy and resource requirements of current labor-intensive manufacturing processes through efficient and compact design, precision-fit materials, fewer manufacturing steps, reduced waste, and on-demand manufacturing. Metal additive manufacturing relies on high purity industrial gases and precision gas mixtures to fabricate products that meet the stringent technical specifications and the required mechanical and physical properties. Gases such as argon are an integral part of every manufacturing step in the metal powder production process — from inert powder storage to inert build chamber atmosphere — and to the final fabricated part post-processing.
Within the world of metal additive manufacturing, powder bed fusion (PBF) is currently among the most widely accepted industrial processes. End markets for PBF parts include automotive, medical devices and implants, defense, and aerospace components. Parts produced with PBF have even landed on Mars. As can be expected with any maturing manufacturing process, increased adoption of additive manufacturing, including PBF, demands consistent part quality, high reliability, and long-term repeatability. Equally important is the cost of production that needs to be comparable or better than current manufacturing techniques. While quality requirements have dramatically increased, total costs such as cost of production, equipment, and consumables have come under intense pressure. Many PBF machine operators, OEMs, and metal powder manufacturers have had to lower their prices to remain competitive, with the understanding that any loss in unit pricing would be gained through higher volume long-term.
Continuous removal of gas and metal contaminants from the build chamber, an essential step in the PBF process, affects both product build quality as well as cost. Up until now, less attention has been paid to industrial gas supply (arguably the second largest raw material cost of the PBF process after metal powder). However, this too is changing, as PBF manufacturers are demanding higher quality gas at a lower cost. As a result, industrial gas providers are innovating ways to meet stringent quality requirements through improved gas handling, while concurrently reducing unit pricing.
Industrial gas manufacturers who can demonstrate value to their customers in the form of higher process productivity, higher material utilization, higher gas use efficiency, competitive gas pricing, and more efficient and safer gas handling will be the ones most successful in helping the industry reach full commercialization.
There are numerous ways that industrial gas providers can add value to their PBF customers to ensure a robust and repeatable industrial manufacturing process. These include efficient build-chamber gas recirculation, improved powder handling using inert gases, specialty powder storage units, improved reliability of supply, gas recycling, and on-site gas generation.
These process improvements will not only reduce failed builds but also result in lower gas cost and less powder waste — major goals for PBF manufacturers. It is important to understand that proper gas handling and recycling will reduce the costs and environmental impact of the PBF process. Over the past several years, leaders in the supply of argon to PBF manufacturers, such as TNSC, have worked to develop successful strategies for sustainable gas usage in (a) new powder production, (b) powder storage, and (c) the PBF process itself. This article provides greater detail on efforts underway in these three areas.
Additive manufacturing uses metal powders as the base material for manufacturing parts in several AM build processes. Metal powders have evolved in the last century to support a broad range of applications in multiple industrial manufacturing sectors. From simple earlier applications in ceramics, paints, and cosmetics they are now sourced for the most advanced applications such as superalloys, sophisticated electronics, and complex medical instruments.
Numerous methods are used to produce metal powders from valuable metals and alloys such as titanium, aluminum, stainless steel, iron, copper, nickel-base alloys, and cobalt-base alloys. Atomization is generally viewed as the method of choice due to the geometrical properties of the powder particles that makes it an ideal choice for additive manufacturing (AM).
Gases in Powder Production
In several metal powder manufacturing processes, gases are used to shear the molten metal into small droplets and to prevent oxidation through an inert atmosphere. In the gas atomization process, a molten metal stream is broken up into small droplets by a high-pressure gas stream in a tower. As the droplets free fall, their surface tension coalesces droplets into solid spheres collected below as metal powder. While any gas can provide the required shear force, the use of nitrogen or argon prevents oxidation. Powder size, shape, and surface roughness can be controlled by adjusting the metal feed rate, atomizing gas pressure and flow, and nozzle design. (See Figure 1)
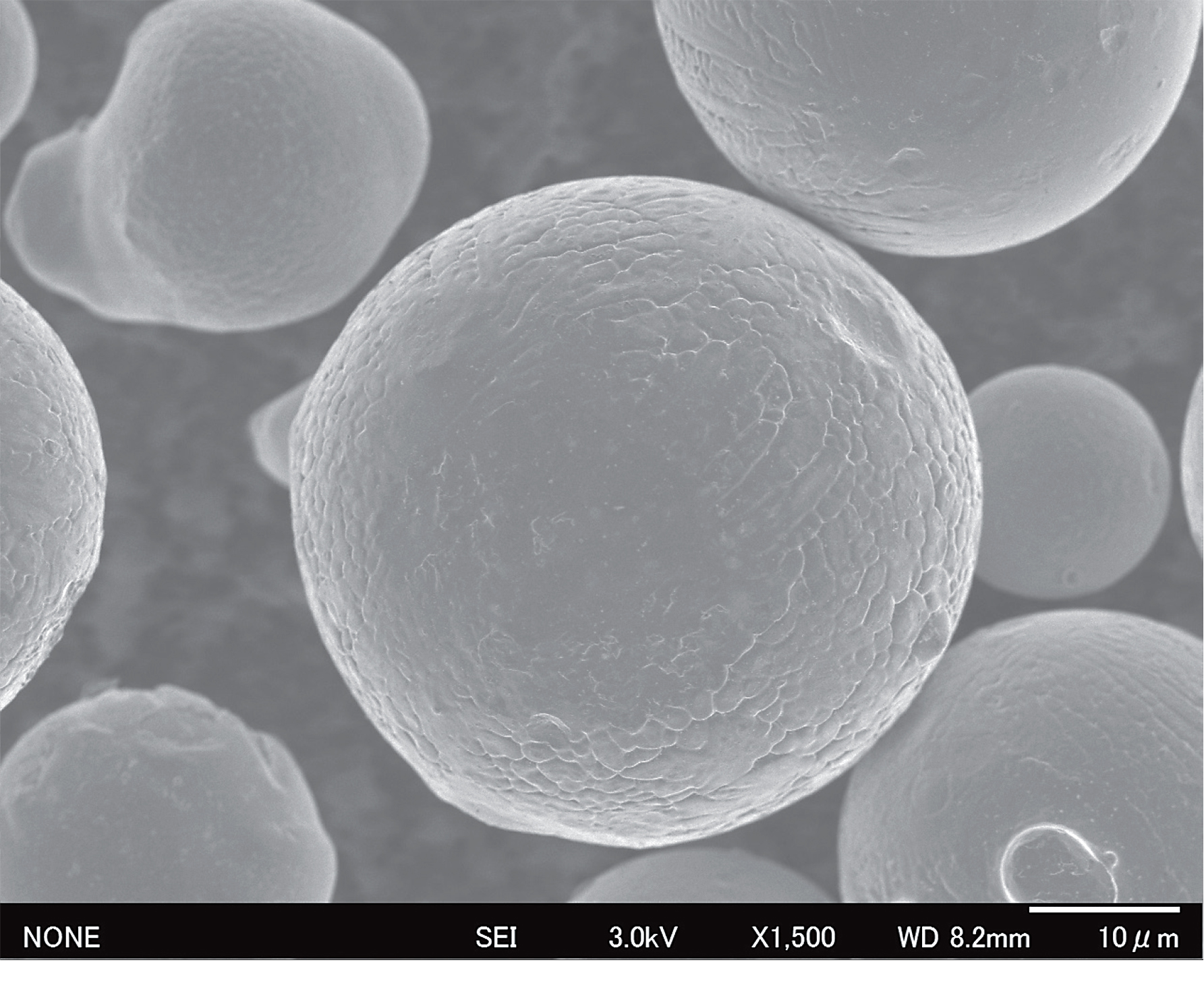
Gas Quality
Building parts in a controlled atmosphere while removing build-process impurities is a major challenge in metal additive manufacturing. Thus, the selection of gas and its supply system are critical factors in the production of powders sensitive to oxidation since the choice of gas atmosphere and the gas purity play a major role in process productivity and powder quality. Contaminated atomizing gas will inevitably result in contaminated powder material.
Gas Recycling
The large volume of gas used in the powders production process creates an opportunity to reduce costs and lower environmental footprint. This is accomplished by capturing and recycling the gas back to the powder atomization tower. Reduced virgin gas requirements lower the energy needed to produce, package, and distribute the gas. However, the gas recycling system needs to be carefully designed. The intent is not only to reduce the net volume of gas consumed in the powder production process, but also to purify the recycled gas to near virgin gas quality. This is critical as even a very small atmospheric contaminant increase can alter the specifications of the powders used in the additive manufacturing machines.
If not caught during quality inspection, these powders can greatly affect the mechanical and/or chemical properties of oxygen-sensitive alloys such as titanium or aluminum. This results in reduced physical properties such as poor fatigue resistance, which is critical for AM growth in numerous industries (aerospace, automotive, medical, etc.).
Gas Recycling System
A well-designed gas recycling system can recycle 80 percent or more (alloy-dependent) of the gas used in the atomizer, while achieving less than 50 ppm total impurity and less than 5 ppm oxygen impurity at parity with “industrial grade” (99.995 percent purity) virgin gas.
System Description
The recycling system captures vent gas from atomizing towers downstream of the atomizer’s cyclone separator and dust collector. A recovery blower facilitates gas transfer from the dust collector to a recovery compressor. The purifiers then remove fine dust and air contaminants in the recovered gas. Later, the high-pressure recycle compressor compresses the purified gas and stores it in the high-pressure tanks for reuse during the next atomizing cycle. The compressors have a fine particulate filter, coalescing filter, and a carbon bed filter for oil removal to 0.01 ppm. (See Figure 2)
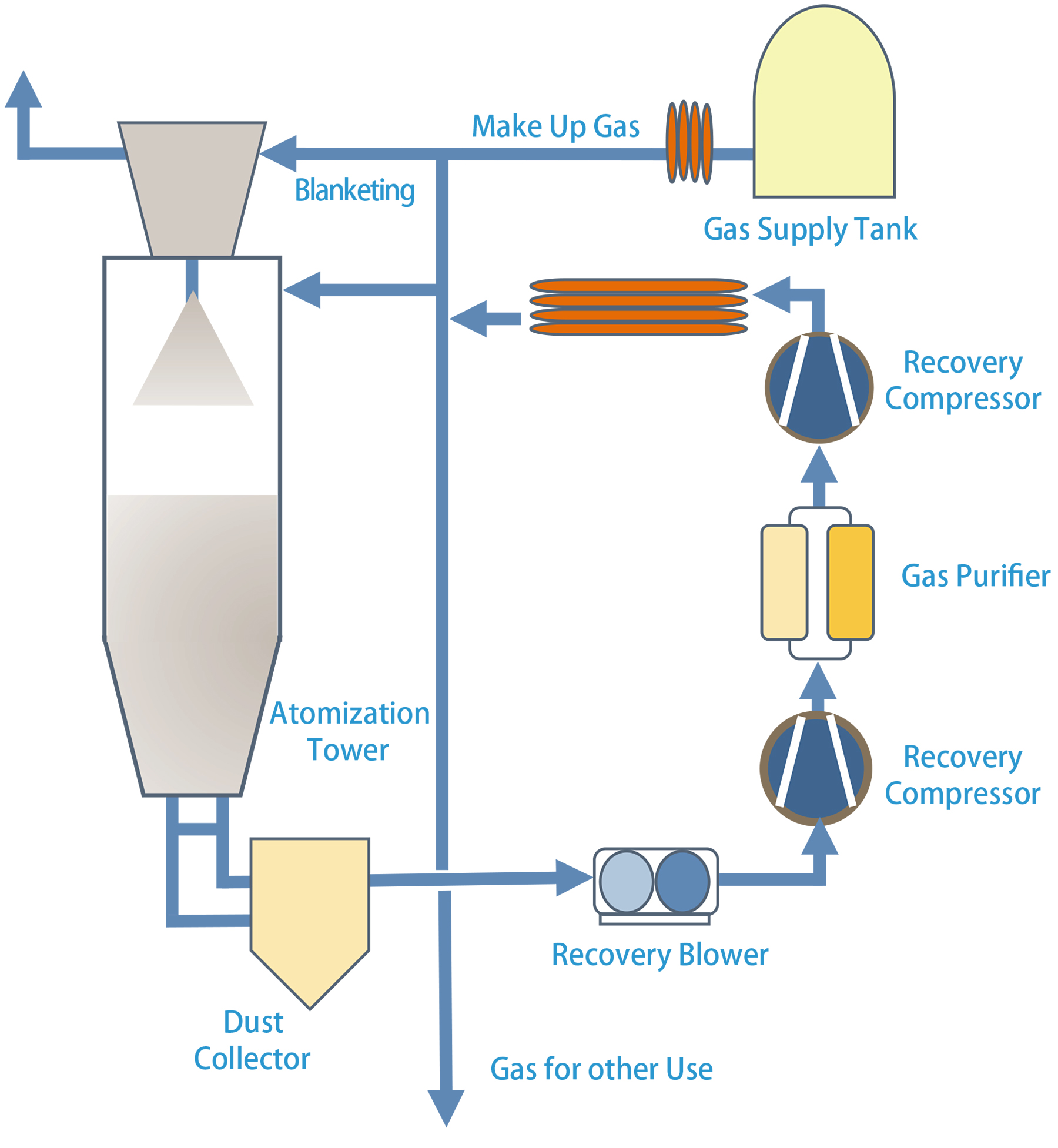
Gas pressure is regulated down to 300 to 500 psi depending on the atomizing process requirement for on-demand gas supply to the atomizing towers. Virgin gas is automatically supplied as needed to make up for gas lost to atmosphere and during the purification process thereby maintaining the required volume of gas supplied to the process.
A gas recycling system reduces gas requirements, and thus its environmental footprint in the form of energy to manufacture, storage and handling systems, and its distribution. It also lowers the cost to manufacture metal powders, contributing to the lower cost of additively manufactured parts and contributing to growth in environmentally greener manufacturing.
A key problem with the current state of metal additive manufacturing is the uniform part quality and part-to-part consistency. Powder contamination is a critical source for contaminants such as oxygen and moisture in additive manufacturing.
Powder contamination occurs during powder handling processes and storage containers. Oxygen and moisture come in touch with the powder from exposure to the surrounding atmosphere. Even with the powder container cap closed tightly, moisture permeates through the container wall to contaminate the powder. To avoid contamination, it is crucial to minimize contact with atmosphere and store powders in sealed containers.
In many climates, the amount of moisture in the air fluctuates greatly throughout the year, with a dew point of below freezing in the winter and 30°C in the summer. If powder is not suitably stored in a tightly controlled environment, the powder’s moisture concentration could fluctuate by the season.
Moisture in powder causes numerous quality effects, including altering the concentration of oxygen in the part, TIP and strength reduction in aluminum alloys, and diminished re-coating property. This can cause a variation in part properties due to season, making it difficult for a production facility to manufacture consistent and equivalent products. To guarantee part quality, it is critical to ensure a suitable powder storage environment.
There are currently no established standards for the oxygen concentration in powders with varying concentration difference between various manufacturers’ samples. In addition, the powder characteristics of different manufacturers are quite different in other ways as well, including a difference in sphericity of approximately 1.5 times. Because powder degradation occurs with powder reuse and its storage environment, even more variation in powder characteristics can occur in reused powders. To stably achieve high-quality printing, it is necessary to be cautious in the selection and storage of suitable metal powder.
Results of the effects on powder flowability are measured using Al alloy powders stored under various conditions as shown as Table 1. There were three types of powder storage conditions: a) immediately after opening the powder container, b) one month of atmospheric storage (with air conditioning management) after opening the container, and c) one month of storage in an inert atmosphere after opening the container. Compared to the powder measured after immediately opening the powder container, the flowability of the powder that underwent one month of atmospheric storage was clearly worse.
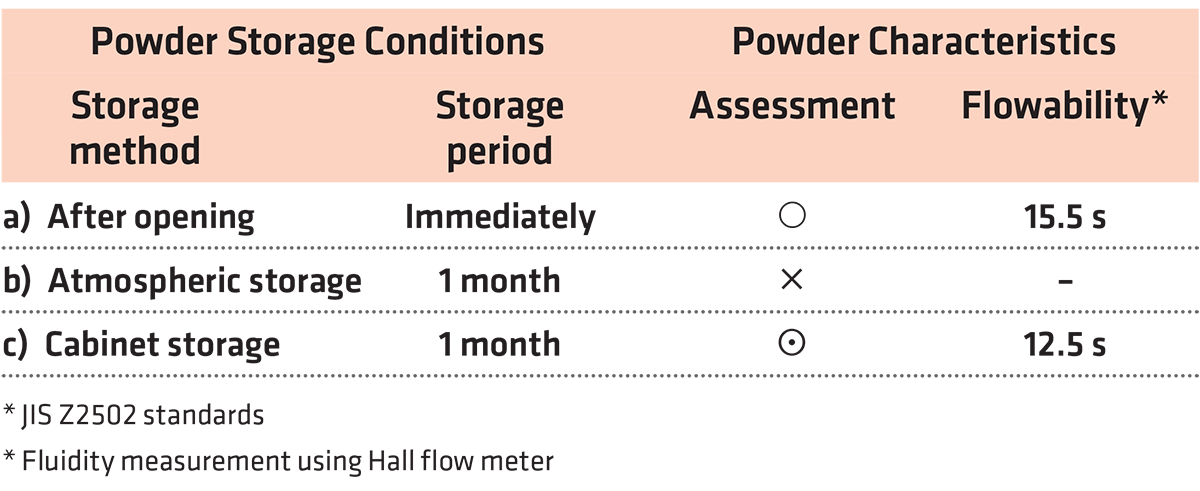
Storing the powder in an inert-gas atmosphere for one month not only maintained the flowability but also resulted in a clear flowability improvement. This is attributed to lower friction between particles with low moisture content of powder as well as less oxide film formation on the particle surface.
Effect of powder storage atmosphere on oxygen and moisture adsorption
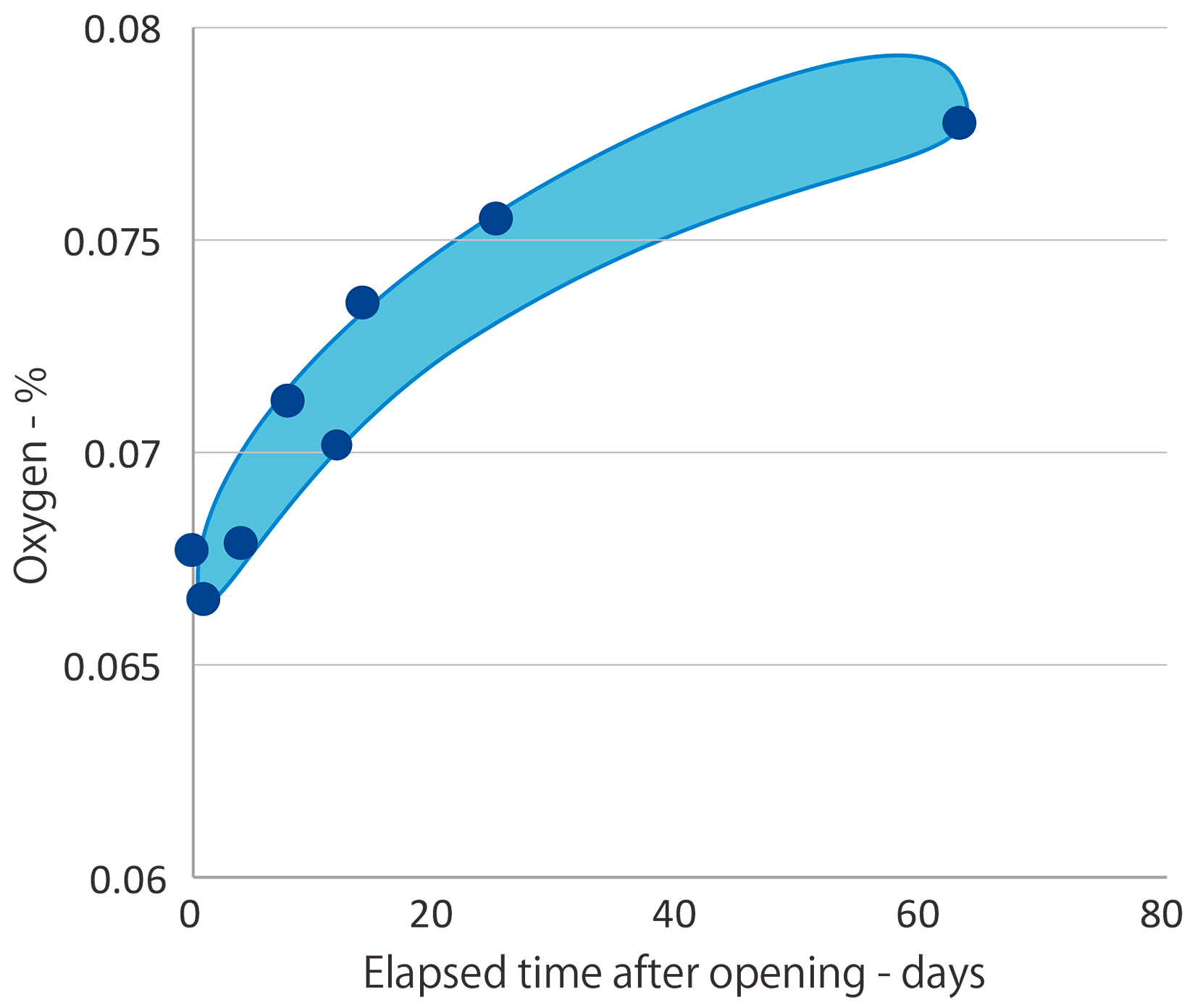
It has been shown that the oxygen concentration of metal powders increases immediately after the container is opened and continues to increase over time. Since powder oxygen concentration is directly linked to metal oxidation, it is important to manage the powder storage environment.
As shown by Figure 3, when a powder container was opened, the oxygen concentration was approximately 0.067 percent. Two weeks later, powder oxygen concentration increased to 0.07 percent and after 60 days, increased to more than 0.075 percent. It clearly demonstrates that atmospheric storage of metal powder increases oxygen adsorption in metal powders.
The moisture content of metal powder also increases when there is an increase in the amount of moisture in the storage environment. Powder moisture content increased by 50 percent when powder was stored for five days in atmosphere with a 10°C dew point. It increased by more than 100 percent when the powder was stored in atmosphere with a 20°C dew point. However, the powder moisture increase was less than 10 percent when powder was stored in an atmosphere with a minus-60°C dew point. It was clear that the powder storage atmospheric dew point has a major effect on powder moisture adsorption.
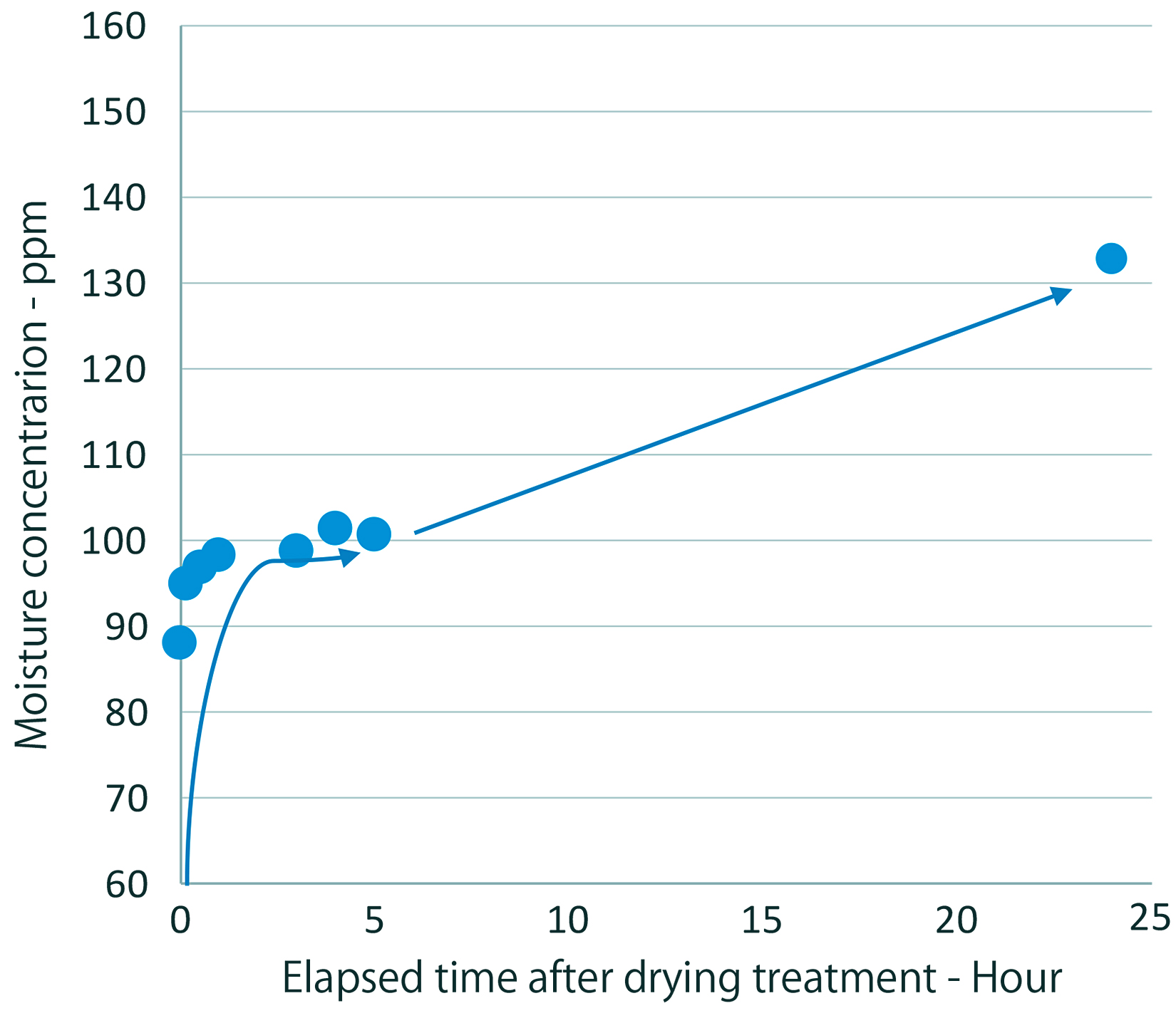
Compared to the powder immediately after it is dried, the included moisture concentration increased by approximately 10 percent after one hour of atmospheric storage and more than 50 percent after 24 hours of such storage, shown in Figure 4. This makes it clear that moisture adsorption occurs quite a bit more quickly than powder oxidation.
Dry Powder Storage Cabinet
It is clearly apparent that clean dry storage of metal powders is a necessity to manufacture high quality and consistent parts, which is required to gain widespread commercial additive manufacturing acceptable to the industry. The risks associated with high oxygen and moisture lead to inferior and inconsistent part quality.
A good dry powder cabinet for AM accurately and automatically manages cabinet oxygen concentration and dew point to provide clean dry storage conditions. This cabinet achieves low oxygen and moisture concentration by continually replacing cabinet atmosphere with low dew point dry inert gas inside the cabinet. In TNSC’s powder cabinet, the concentration of oxygen inside the cabinet is less than 1 percent after one hour of storage, which is eventually reduced to 20 ppm. The moisture inside the cabinet is a minus-30°C dew point (375 ppm) after one hour of storage, which can eventually be reduced to about a minus-70°C dew point (2.58 ppm). (See Figure 5)
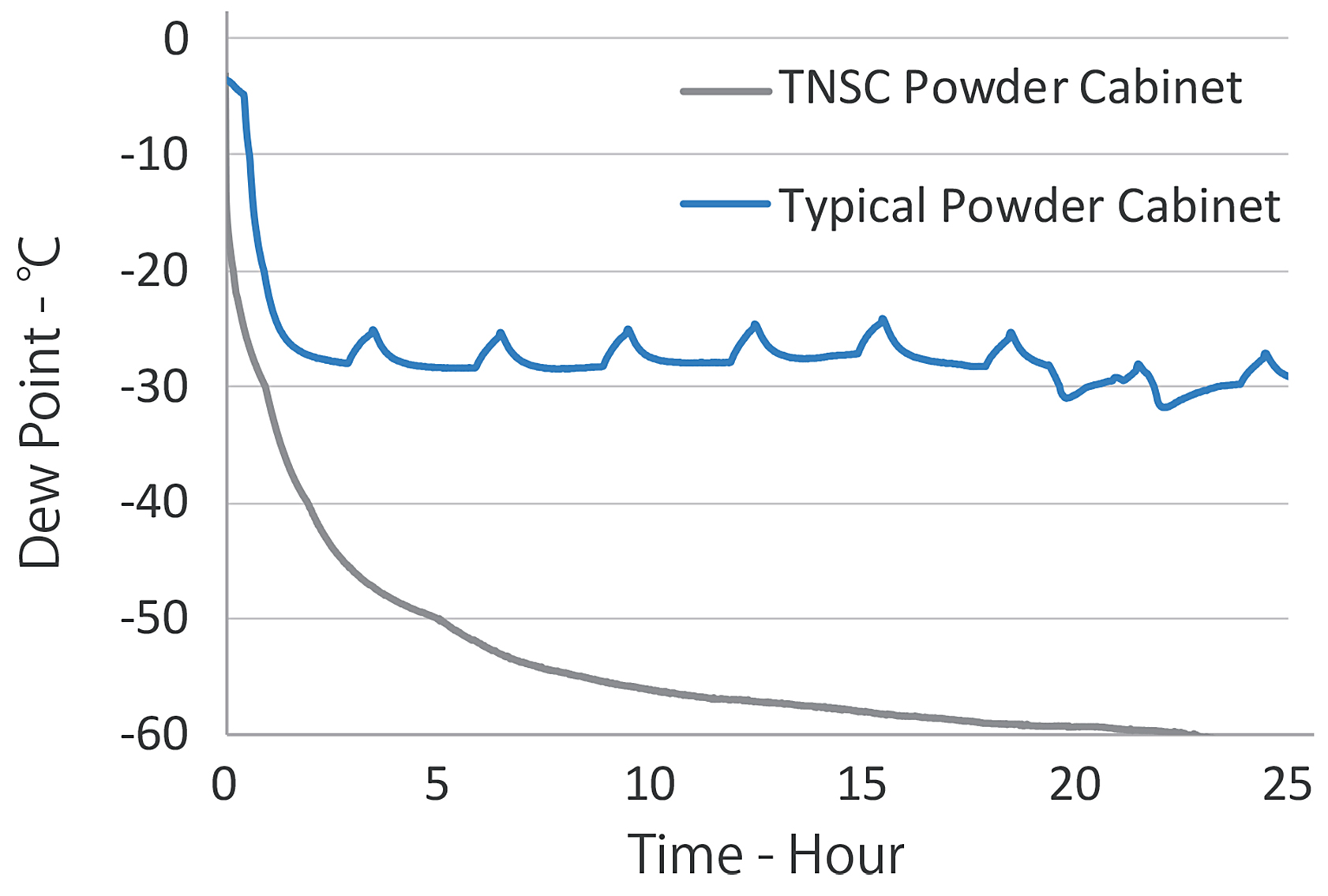
TNSC dry powder cabinet is also superior to conventional powder storage cabinets in terms of achieved dew point and the speed of dew point reduction. In addition, the dew point of an unopened polyethylene metal powder bottle stored in TNSC dry powder cabinet for two weeks and then stored in a 20°C dew point atmosphere for two weeks after that. Storing the powder container in a TNSC dry powder cabinet reduces the container’s internal dew point and is effective for preventing the deterioration of powder characteristics. Conversely, when the powder container is stored in uncontrolled atmospheric conditions, permeability occurs, and the powder characteristics deteriorate even in sealed containers. (See Figure 6)

Contaminants in the part-building process are one of the main causes of defects in built parts in metal additive manufacturing. In powder bed fusion (PBF) additive manufacturing machines, contaminants such as oxygen, nitrogen, and moisture enter the build chamber through the addition of metal powder. Part of the contaminants come adsorbed on the powder particle surface while others come through the machine’s powder feed entry. Typical PBF print chamber atmospheres contain more than 1,000 ppm of impurities, mainly as oxygen and moisture. As mentioned previously, these impurities contribute to deterioration in various printing part properties.
Effect of build chamber gas impurities on spatter
As the concentration of oxygen in the atmosphere build chamber was increased, the amount of spatter increased. Although there are reports that spatter can be managed with laser control, the operational parameters may not necessarily match the process conditions for specific parts to eliminate it. It is therefore important to control the chamber atmosphere to control spatter. (See Figure 7)

Effect of build chamber gas impurities on porosity
Similarly, oxygen and moisture contamination in the build chamber affect the porosity of the part. Figure 8 shows how inert gas atmospheres under different oxygen concentration and moisture concentration affect porosity in a typical PBF printer.
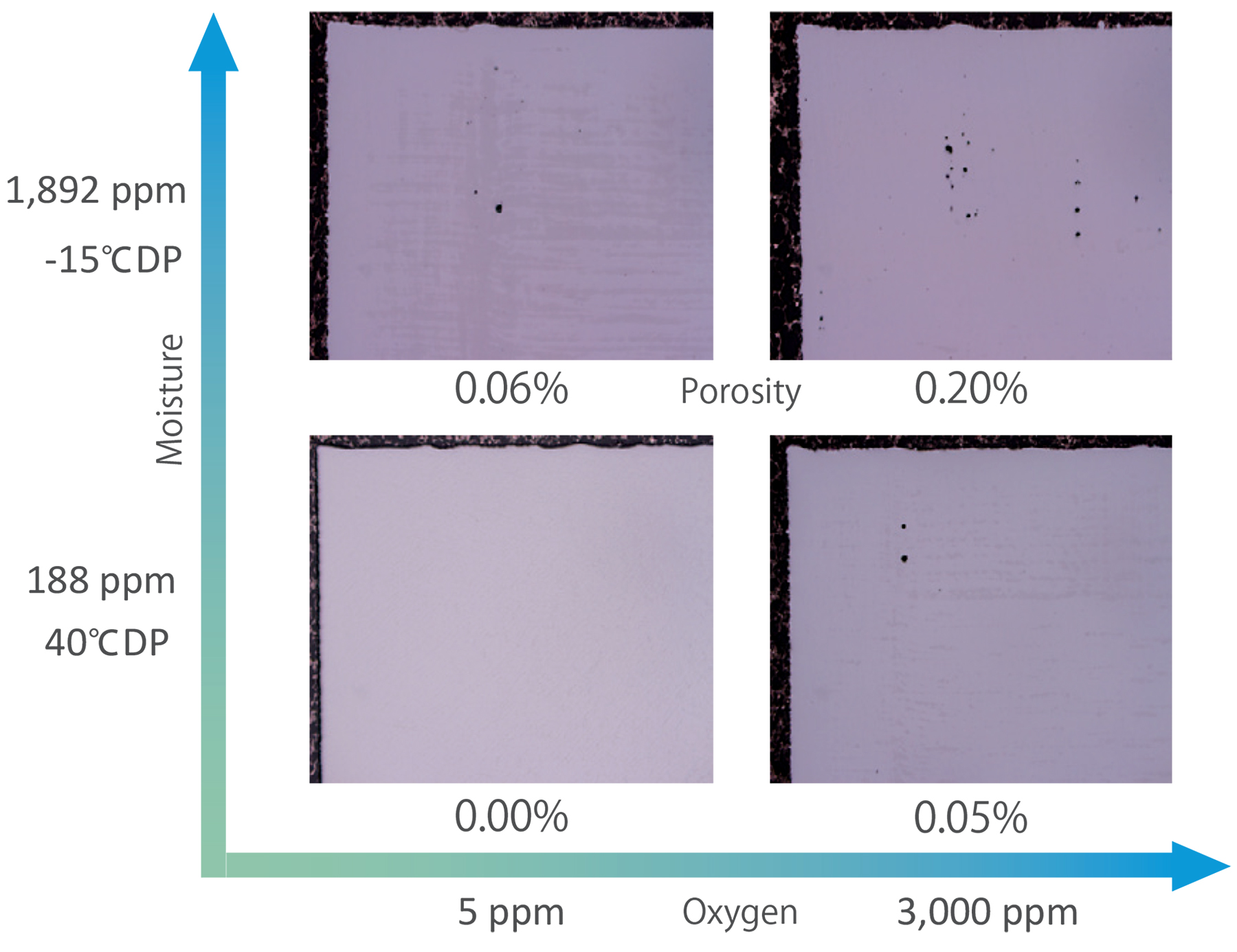
The porosity in a part increases with the increase of either the oxygen or moisture contamination. Even higher porosity is seen when both the oxygen and moisture are high. Impurities in the printing atmosphere cause increased spatter and fumes, laser attenuation, and scattering. So, reducing impurities in the printing atmosphere is critical to reducing part porosity and improving its relative density.
Additionally, when the concentrations of oxygen and moisture in the printing atmosphere increase, the part-internal oxygen concentration increases. Surprisingly, TNSC confirmed that the concentration of moisture in the printing atmosphere has a greater effect on the part-internal oxygen concentration than the oxygen itself. Since oxygen concentration must be strictly regulated in titanium alloys Grades 5 and 23, the effects of impurities in the printing atmosphere become even more crucial. In addition, increases in fumes or spatter also affect the concentration of oxygen in the recycled powder as well as the recyclability of the powder. From the perspective of metal oxidation, it is clearly necessary to manage both the oxygen and moisture in the atmosphere.
Effect of build chamber gas impurities on Blister
and Thermal Induced Porosity (TIP)
One typical defect of aluminum and aluminum alloys is the occurrence of blisters on the alloy surface swelling after heat treatment. Blisters are surface defects caused by the internal pressure of inherent hydrogen and hydrogen absorbed from the atmosphere. In addition, even in cases where the problem does not advance to surface swelling, TIP micropores often occur because of heat treatment and reduce the mechanical characteristics of alloys.
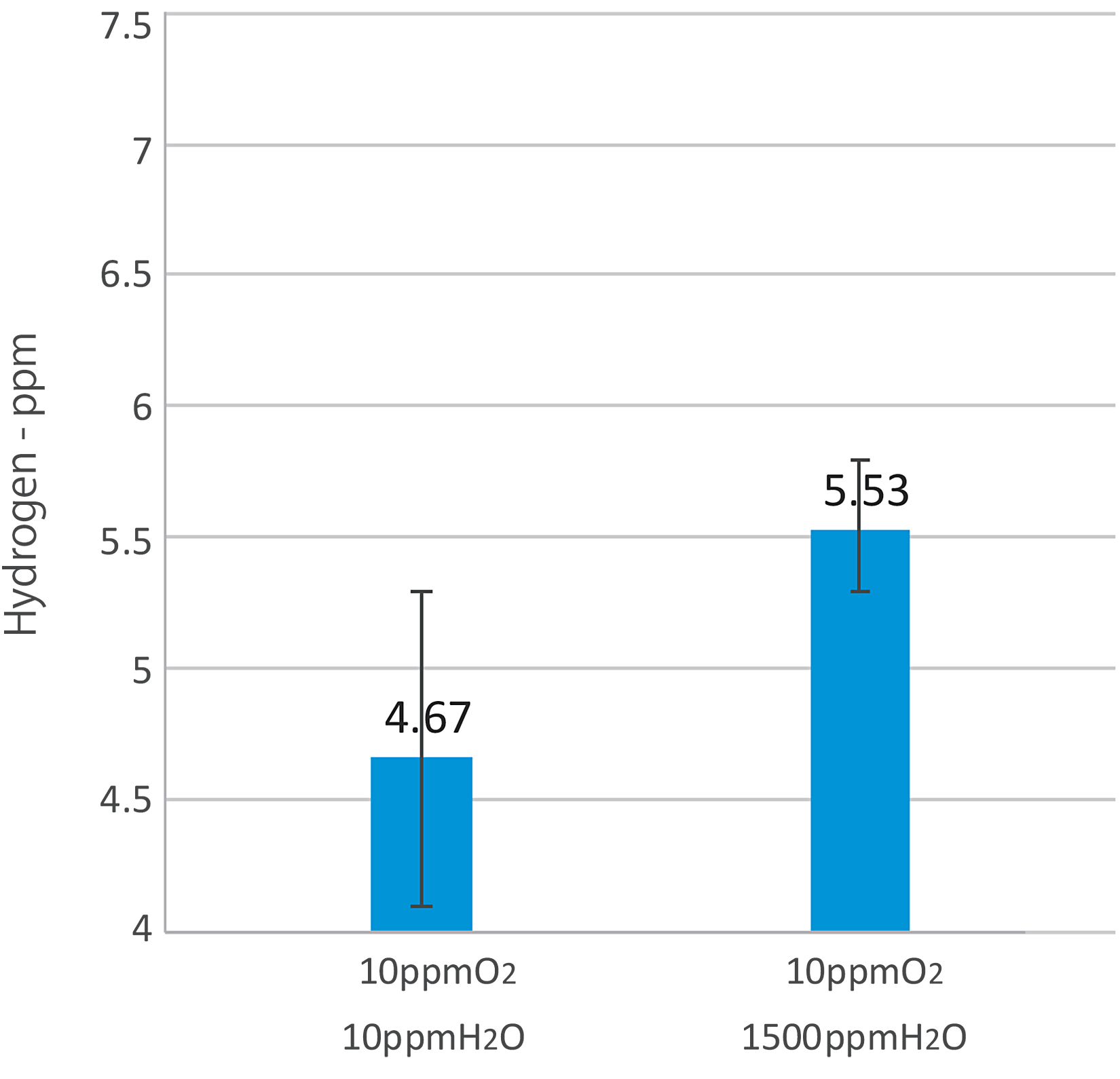
Our research shows that it is hydrogen that causes TIP in the part. The more impurities in the printing atmosphere, the more hydrogen on the part. Hydrogen increased approximately 20 percent when the chamber moisture was increased, showing moisture has an especially strong effect. When the powder was dried, the included hydrogen concentration decreased by 33 percent. Based on this data, it can be concluded that the moisture in the printing atmosphere and moisture that gets on the metal powder results in the generation of hydrogen. Since hydrogen can cause delayed fracture in most metals, it is extremely important to manage moisture in the atmosphere as well as moisture in the powder. (See Figure 9)
The Advanced Build Chamber Gas Purification System
It is evident that any contaminants in the build chamber can cause part quality and part-to-part consistency problems. These contaminants continually enter the chamber as part of the powder or through the powder-feed openings and must be removed continuously as the part is built. TNSC’s 3DPro® PrintPureTM addresses the problem by collecting, purifying, and re-circulating the printing atmosphere gases. (See Figure 10)

This system is an add-on gas recirculation and purification system for PBF printers. Circulating and refining atmosphere gas in a printer makes it possible to remove the oxygen and moisture down to low ppm levels.
The system can additionally include the proprietary Nanochem® unit that makes it possible to remove oxygen, moisture, and other impurities in the supply gas line and provide ultra-high purity gas to the chamber. A Nanochem® unit can easily be installed in a gas supply line. In the welding industry, a key field of expertise, TNSC has extensive experience using similar inline gas purifiers all over the world.
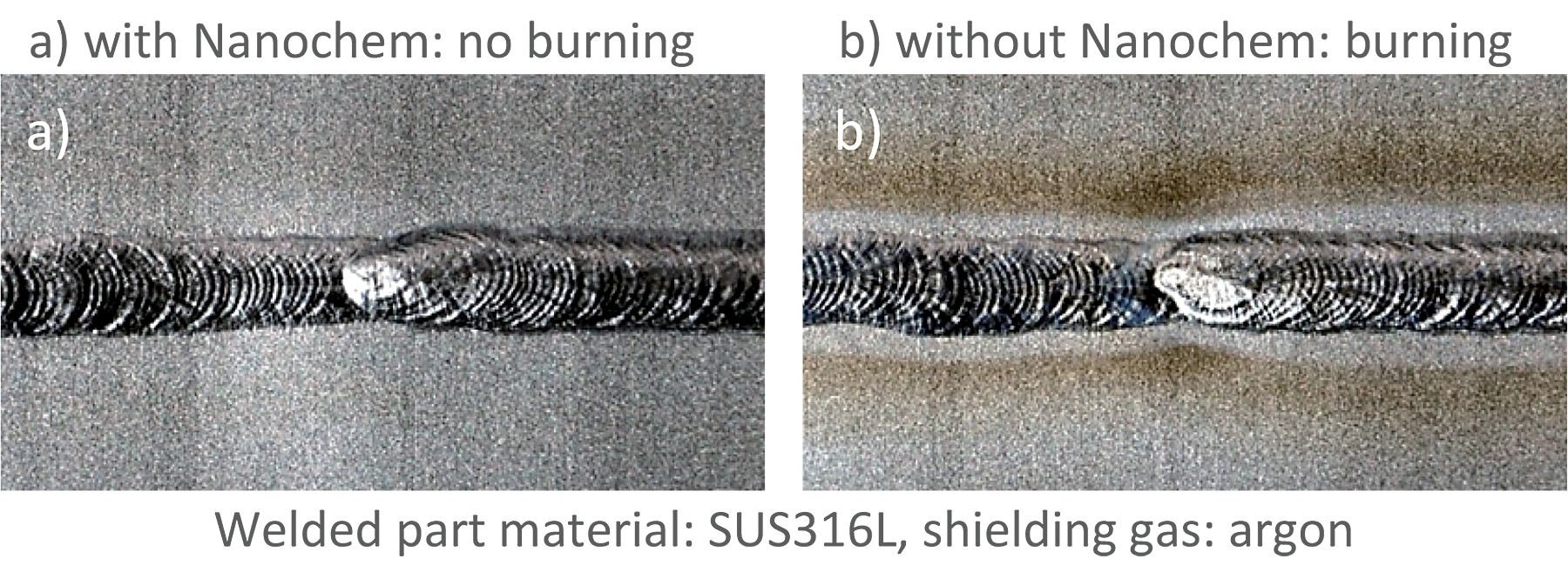
Figure 11 illustrates the big difference in a welded part burn with and without the use of the gas purification system. When Nanochem® purification was not used, the gas dew point was -37.9°C, and there was a lot of welded part burn. In contrast, when Nanochem® was used, the gas dew point was reduced to minus-81.6°C, an extremely low dew point, and hardly any gas burn was noticed. Reduced oxidation also improves the print part characteristics, so Nanochem® is valuable not only to additive manufacturing atmosphere gas but also for the shield gas in WAAM and DED technologies.
Conclusion
The importance of employing sustainable gas strategies in the production cycle of the PBF process is recognized by industry leaders such as TNSC, which have employed them in the production of metal powder, storage of metal powder, and printing of final components, further reducing the environmental footprint of the additive manufacturing process. These strategies have allowed for an improved industrial ecology of the PBF process and can be leveraged and improved upon as the industry continues to grow.
This article reprinted courtesy of Taiyo Nippon Sanso Corp. (TNSC). TNSC was founded in 1910 and based in Tokyo, Japan; it is a global supplier of industrial gases. TNSC is a “single source” for industrial, welding and safety supplies, medical, specialty and electronic gases, gas handling equipment, high performance purification systems, engineering and gas management services, as well as onsite gas generation. Its mission is to deliver innovative solutions that meet global customer requirements. TNSC is one of the five largest suppliers of industrial, specialty, and electronics gases in the world. The Additive Manufacturer Green Trade Association (AMGTA) is a global organization with the purpose of promoting environmental sustainability with AM technology applications. TNSC is a founding member of AMGTA and cooperates with AMGTA companies, research institutions, and industrial organizations to promote education, enlightenment, and research.





































