Quenching of various alloys to achieve mechanical properties has been an area of scientific research for many years. Using polymer quenchants has proven to be one of the best methods to achieve mechanical properties due to its flexibility and safety. There are three major stages of polymer quenching: vapor phase, boiling phase, and convection phase. What happens in these three phases will determine the mechanical properties of the material.
The vapor phase is the first major stage, which happens when the part enters the quench medium (water, polymer, oil, etc.). As soon as the part enters the quenching medium, the metal is surrounded by a thin film around the surface. Since the liquid is a cooling medium, there is heat transfer at this point. The heat is transferred through radiation and conduction during the vapor phase. The latent heat of vaporization is critical at this stage.
The boiling phase is the second major stage and follows immediately after the vapor phase. The vapor film disappears in the beginning stages of the boiling phase. The disappearance of the vapor film allows the quench medium to come in contact with the hot metal being quenched, causing the surrounding quench medium to boil. During this phase is when the highest heat extraction rate occurs. The surface tension is critical and the quench medium properties become a factor.
The last major phase is the convection phase. During the convection phase, the boiling has completely stopped. As the name of this phase states, the heat is removed slowly by convection into the liquid. The rate of convection cooling is determined by the liquid properties only, and the viscosity of the medium is critical.
Quench mediums
There are three popular quench mediums: water, oil, and polymer quenchant.
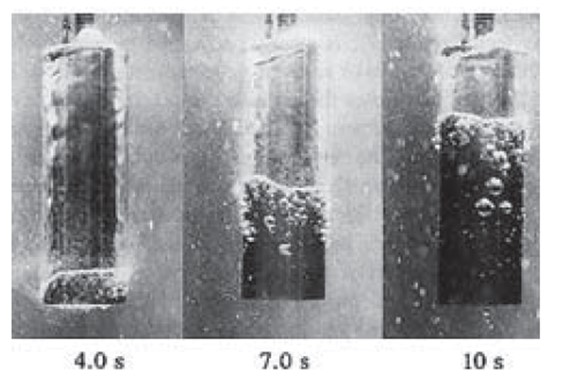
Water has the highest cooling rates, which range between 2,000°F/sec to 10,000°F/sec. The vapor film phase depends primarily on the surface finish (Figure 1). The cost is inexpensive, easy to maintain, and has a very low safety risk. Water offers very high flexibility, being that only temperature can be adjusted to change quench characteristics. Due to the variation in cooling rates, the parts exhibit the highest distortion and cracking rates of all mediums. Soft spots on the component surface can develop due to an increase in temperature in the water, causing a longer vapor phase. The high cooling rates on steels during the martensitic transformation temperature will result in high residual stresses, excessive distortion, and increase the probability of potential cracking. From a maintenance perspective, there is a high potential for bacterial growth if not properly agitated and filtered.
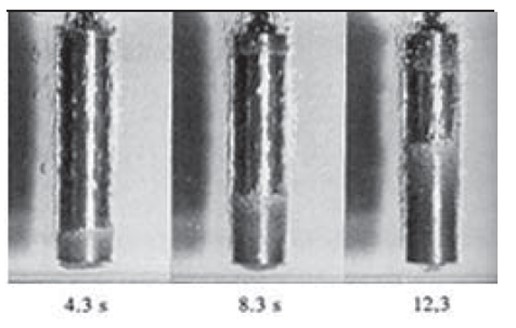
Oil is considered a favorite of the steel industry (Figure 2). Oil quenches can be found in three categories: normal, medium, and high-speed grades. Normal speed quench has a slower rate of cooling, thus, alloyed material and tool steels are typical. Medium speed quenching oils are used when medium to high hardenability is required. High speed quenching oils are used for carburized and carbo nitriding applications. Oils are favorites when large, thick parts are required. Although very popular, there are some disadvantages. Parts that have been quenched in oil have a need for alkali cleaners or solvent degreasers. There are also significant safety risks. Water must be avoided as much as possible. Less than 1 percent of water can have an effect on soft spots, distortion, and cracking. The foaming of water molecules during quenching can cause fires or explosions. Sludge content in the tanks can cause part staining and changes in mechanical properties. Oils also exhibit a change with usage as well. Oils with use have a loss of volatile components, oxidation of compounds, depletion or loss of additives, and thermal degradation.
Polymer quenchants are a hybrid of both water and oil. They are soluble in water and are clear at room temperature. As an aqueous solution of a liquid organic polymer, it can come with a corrosion inhibitor. When shipped from a manufacturer, there is 40- to 50-percent water in most occasions. Only water needs to be added to a system to get the required concentration. In general, they have a higher molecular weight, higher viscosity, and lower thermal/oxidative stability.
There are various polymer quenchants, including Polyalkylene Glycol (PAG). PAG is unique in that it can be separated. This is possible because water hydrogen bonds with the PAG, giving the mixture seen in a tank. When a PAG is taken above 167°F, the hydrogen bonds are disrupted, causing cloudiness that is referred to as the cloud point. The mixture at this point is in two phases.
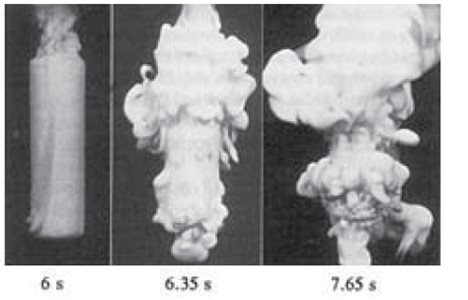
Looking at three stages of quenching for a PAG will give insight on how a polymer quenchant is unique and flexible (Figure 3). A water vapor blanket will form around the part when quenched, as in any other medium. A PAG can have different rates of diffusion of water to surface based on the concentration. When the temperature of metal drops and the PAG heats up, the boiling phase and the critical heat flux begin. At this point, the polymer layer is disrupted, which allows a water rich concentration to interact with the surface of the metal. After the metal temperature has dropped below boiling, some of the liquid around the metal may still be above the cloud point. This leads to a high viscosity boundary layer allowing for convection heat transfer.
There are several factors that affect the heat transfer rate in polymer quenchants to the parts. The quench concentration plays a major role. The higher the concentration, the slower the cooling due to the viscosity and film thickness. The thicker the film, the harder it is to break the film, allowing the part to cool for a fraction of a second longer before the water hits the part. Agitation of the quench tank also plays a major role (Figure 4). Agitation reduces the thermal gradients and has direct correlation on timing of film rupture/breakage. The higher the agitation, the faster the cooling. Temperature control is also critical. Higher bath temperatures will lower the cooling rates. For aluminum, it is very common to see the temperature inside of a tank between 70°F to 90°F where higher cooling rates are acceptable. This is in contrast to steel, where the temperatures tend to be between 90°F to 140°F.
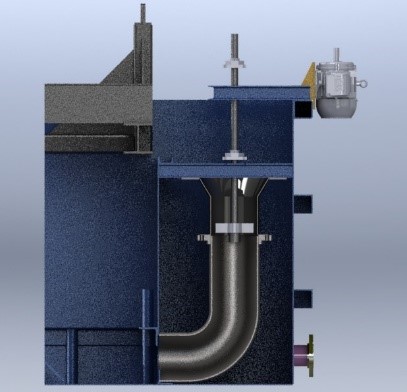
Polymer quench tanks require maintenance and checks that water and oil do not. Daily concentration analyses are recommended and, in some cases, required per specifications. Concentrations are performed by a BRIX refractometer (Figure 5). A BRIX refractometer displays readings in the BRIX scale. BRIX is a unit of measure, which is related to the sugar content of a sugar solution and can be used for quenchants. All manufacturers will provide you with a chart to determine the concentration of your tank based on the reading. Quarterly testing by viscosity is recommended to compare with daily numbers. Large differences between concentration by BRIX method and viscosity indicates a problem with the polymer quenchant. It could mean contamination or polymer degradation. Periodic bacterial dipsticks can be used to minimize the risk of bacterial growth. The beauty of using a polymer quenchant is that if you have hydraulic fluid or other oil contaminants enter the bath there is a possibility that you will not need to drain the tank. The agitation system can be stopped and when all settles the oil can be skimmed from the tank. Filtration on the tank is similar to that of water, where a bag filter of at least 50 microns will prevent solids from being agitated in the tanks.

PAG quenchants allow for thermal separation. This process is done to remove water-soluble impurities such as salt contamination, degraded polymer, or foaming agents. Thermal separation is performed by taking the bath to 180°F and agitating the tank. After being at temperature for about an hour, turn off the agitation and heat. Let it sit for a while. The tank will be left with a water layer on top and PAG layer on bottom. Pump off the upper layer (water) and add water/PAG as needed to return to desired concentration. On large tanks, the entire solution is pumped out and separated in holding tanks, where it is then sent back as reclaimed glycol.
Polymer quenchants and safety
The unique and most appealing part of polymer quenchants is the safety. Most PAG quenchants have FM approvals. A common type of PAG used in the industry is a Tenaxol UCON A. It has an FM rating of zero and a National Fire Protection Association rating of zero for health, flammability, and reactivity. This in turn reduces risk, which can lower insurance premiums. This is the major advantage and the main reason many shops are doing conversions of oil systems to polymer quenchants. Compared to oil, there are no fire hazards, no soot, and no smoke. Environmental safety is also significantly increased; in the event that there is a spill, it can be easily cleaned up.
Compared to oil quenchants, polymer quenchants do require slight upkeep. Tank agitation and bath temperature require better analysis for initial set-up. However, due to advances in technology, the upkeep is almost neglible compared to the safety risks involved with oil.
The old method of manually logging through BRIX method described above is slowly becoming obsolete. A recording device is continuously recording concentrations. If the concentration gets above or below a baseline, the system will force an alarm to go off. This method ensures that the concentration is correct throughout the day. Using a tank farm and thermal separation, the tank can be cleaned out. With a touch of a button, a tank can be drained, separated, and replenished automatically. A PLC will add water and glycol as needed to replenish the tank when maintenance or clean-out is performed. This is extremely helpful to commercial heat treaters who run multiple parts that need multiple concentrations and agitation speeds. Agitators that are running on variable frequency drives can be easily changed with simple PLC programming. Prior disadvantages for polymers are proving to be advantages, due to greater flexibility of its use (Figure 6).
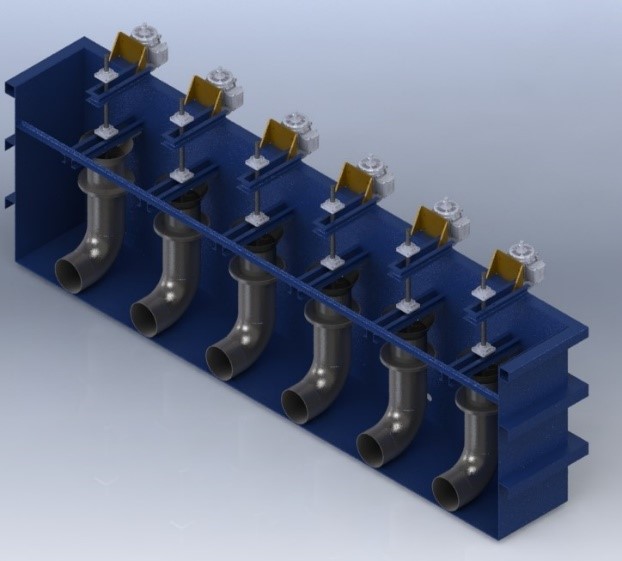
As the industry continues to grow, the use of polymers will grow alongside it, not only for its flexibility for desired mechanical properties, but, more importantly, for safety to the workers.