
In this column, I will discuss quenching aluminum from molten salt into a polymer quenchant.
A molten salt bath is a common method of heating aluminum components to the solution heat-treating temperature. The salt is heated to the solution heat-treating temperature, and parts are immersed in the bath. The salt is usually a mixture of sodium and potassium nitrate, and usually complies with MIL-S-10699 Type I or II compositions (Table 1).
Parts are solution heat treated in salt for many different reasons — because the part is big, because of rapid heating, or cost. After solution heat treatment, the part is removed from the molten salt using some sort of crane, and the part is lowered into the quench tank containing the polyalkylene glycol (PAG) solution. This typically must be done in under ten seconds or less to minimize part cooling. As described in an earlier column, polyalkylene glycol solutions are used effectively to control distortion and residual stresses in many different aluminum product forms (extrusions, castings, forgings, cast, sheet metal, etc.). The molten salt is very liquid at the solution heat-treatment temperature of aluminum, and depending on the geometry of the part, significant drag-out of the molten salt can occur.
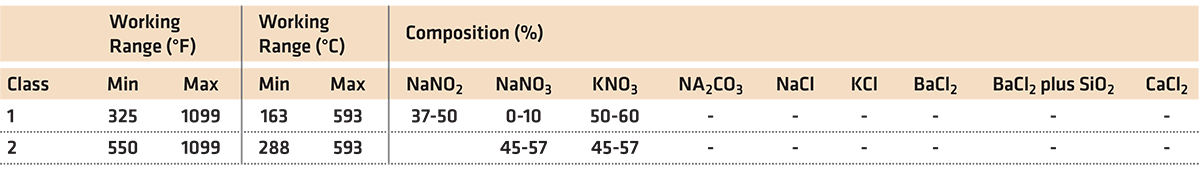
If the solution was strictly water, the specific gravity of the water and molten salt (50% NaNO3 and 50% KNO3) would increase. This increase is linear with the percentage of sodium nitrate and potassium nitrate present in the water (Figure 1). The solubility of sodium nitrate is 848 g/L at 25°C, and the solubility of potassium nitrate is 3830 g/L at 25°C. These salts are both very soluble in water.
Typical polymer quenchants have a specific gravity of approximately 1.09. However, the diluted solutions have a much lower specific gravity of approximately 1.015 at a concentration of 20 percent.
Polyalkylene glycol quenchants exhibit inverse solubility in water. PAG quenchants are completely soluble at room temperature but become insoluble at elevated temperatures. This phenomenon provides the unique mechanism for quenching hot metal by surrounding the metal piece with a polymer-rich coating that serves to govern the rate of heat extraction into the surrounding aqueous solution. The inverse solubility of PAG quenchants occurs at approximately 165°F, known as the cloud point.
If the water and PAG quenchant are heated to above the cloud point, the polymer and water separate, with the polymer sinking to the bottom of the beaker (or quench tank). This is due to the density differences between water with a specific gravity of 1.0, and the polymer quenchant with a specific gravity of approximately 1.09.
However, as the salt content of the water and PAG solution increases, the cloud point of the polymer quenchant decreases. This is mainly a function of inorganic content of the water/polymer solution and specific gravity. If the cloud point decreases to approximately room temperature, the polymer and water solution will separate, with the polymer now on the top of the bath, and water on the bottom. This reversal is due to the specific gravity differences between pure water and water containing the molten salt. This can result in an aluminum part being quenched through a layer of 100 percent polymer, and into the lower layer of water. This lower layer of water has a much faster quench rate due to the presence of salt. This will occur at approximately 8-12 percent salt content.
 Corrective Action
Corrective Action
From AMS 2770N [2], paragraph 3.4.9.4 Salt Contamination:
“Salt content in polymer/water quenchants shall not exceed 6.0 percent by weight. Water/polymer quenchants used with salt bath furnaces shall be tested for salt content weekly. The method used shall be calibrated against solutions containing known amounts of both polymer and salt, and the procedure documented. Meters used to determine the salt concentration shall be calibrated every 90 days. Quench tanks which exceed 6.0 percent salt content shall not be used until the salt content has been reduced below 6 percent or the quenchant has been replaced.”
There are two methods to determine the amount of salt present in a polymer quenchant bath. The first method is conductivity of the solution, and the other method is using specific gravity.
A conductivity meter is used, and consists of a probe and, usually, a handheld display. The probe is placed in the polymer solution, and the meter applies voltages between two electrodes inside the probe. The conductivity of a solution is measured in micro-Siemens or mS. The conductivity of the solution depends on the concentration of the ions present. Usually, some sort of temperature compensation is needed. Measuring at a constant temperature of 25°C provides consistent reading. Meters are usually calibrated using standards available from the conductivity meter supplier.
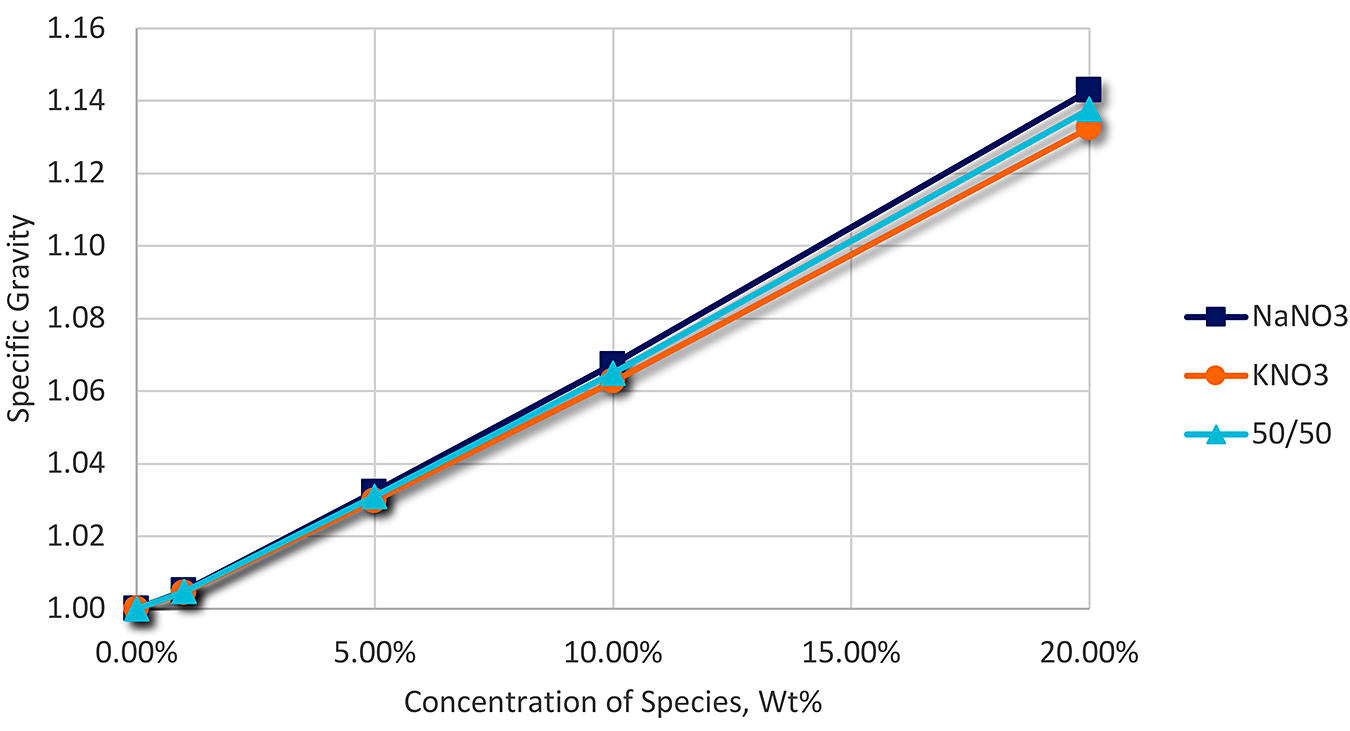
As the salt content increases, the conductivity of the solution also increases. This is generally linear for salts associated with molten salt (KNO3 and NaNO3). This is shown in Figure 2.
At a conductivity of approximately 4000 mS, the salt content is approximately 6 percent, and time to perform corrective action.
Specific gravity can also be used to determine salt content of solutions. Specific gravity of a liquid is measured using a hydrometer. These simple devices, based on Archimedes’ principle, consist of a hollow tube that is weighted at the bottom, and a long stem that is graduated in specific gravity. To conduct the test, a graduated cylinder is filled with approximately 100 ml of polymer/water solution, and the hydrometer placed inside the graduated cylinder. The lower the specific gravity of the fluid, the deeper the hydrometer sinks. The specific gravity of the solution is read from the stem of the hydrometer. Hydrometers are available inexpensively from chemical supply houses.
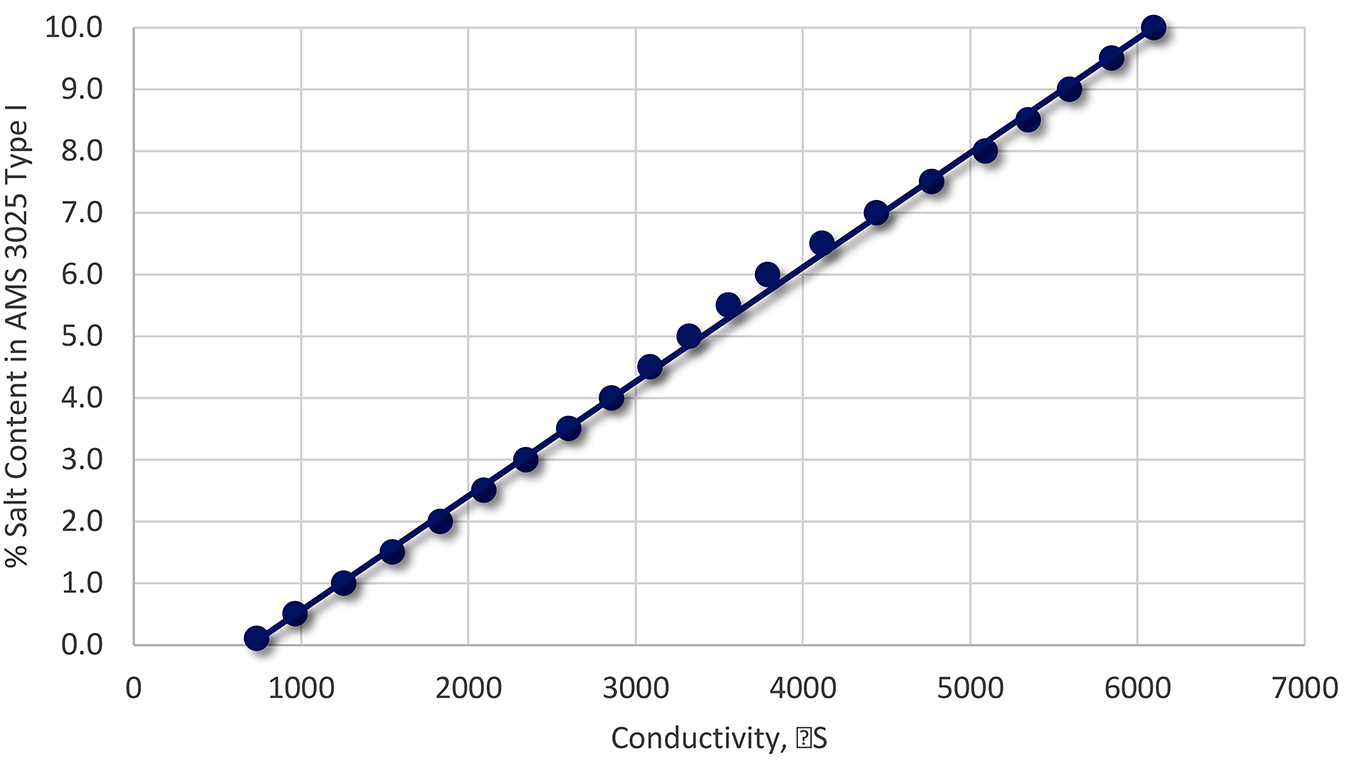
The specific gravity of the polymer/water will increase linearly as the content of salt increases. This is shown in Figure 3.
Should the specific gravity of the solution exceed approximately 1.05, then corrective action is needed. This specific gravity value can change depending on the concentration of polymer present. However, for most installations, this value represents a typical number.
To eliminate the salt content, there are two things that can be done. The simplest is to dump and recharge the quench tank. However, this is not usually cost-effective. The second alternative is to thermally separate the water and polymer.
Performing a thermal separation of the water polymer solution requires heating the solution to above the cloud point, turning off the agitators, and allowing the polymer to separate from the water. Depending on the relative specific gravity (and salt content), the polymer may be on the top or bottom. The inorganic salts report to the water phase. The polymer can then be recovered by pumping it to totes or drums. The water containing the salt is then disposed. The polymer is reused, and new polymer added to the tank to meet the desired concentration. New water is then added. In very sophisticated operations, the water containing the salt is heated to boiling, and the water recovered using a retort. The salt deposits on the retort, and can then be completely reused.
Conclusion
In this article, the problems of excessive salt in a water/polymer solution were explained. Methods to measure salt concentration were detailed, and methods to recover the polymer from a high salt-containing solution were described.
Should there be any questions regarding this article, or suggestions for additional articles, please contact the author or the editor.
References
- “MIL-S-10699B, Military Specification: Salt, Heat Treating (for Metals),” 1977.
- SAE International, “Heat Treatment of Wrought Aluminum Alloy Parts,” SAE International, Warrendale, 2015.
- G. E. Totten and L. C. Canale, “Polymer Quenchants,” in Encyclopedia of Materials: Science and Technology, Elsevier, 2005, pp. 1-11.

























