Heat-treating processes under atmosphere drive desired metallurgical results to meet part performance. When it comes to gas atmospheres for neutral, protective, or carbon-rich atmospheres, there are many assumptions that are made about how the parts are being treated metallurgically, based on the equipment being used for monitoring the atmosphere or incoming gases.
One aspect of understanding what is happening to a part is to consider the surroundings that the parts are exposed to at heat-treating temperatures. You can make many assumptions about the atmosphere based on gas flows and the sensors being used to continuously measure. Temperature and time are, of course, significant to how parts are ultimately heat-treated, but here we will review the gas composition for certain heat-treating atmospheres.
Most atmosphere furnaces operating at slightly above atmospheric pressure with a continuous supply of a carrier gas have some sort of in-situ monitoring. Common applications for this would be annealing, neutral, or a carbon-rich atmosphere. An oxygen sensor is commonly used for in-situ measurement and, with the assumptions of the carrier gas (endothermic gas for example), a carbon potential for the atmosphere can be determined. Carrier gas mostly consists of two variations: nitrogen methanol or endothermic gas.
Nondispersive infrared (NDIR) analyzers are the “go-to” tools for the evaluation of furnace atmospheres. Other tools can be used when trying to evaluate if the atmosphere is correct to achieve a neutral or carbon-rich atmosphere, but NDIR provides an evaluation of three components of the atmosphere that can drive consistency and troubleshoot equipment and gas atmosphere delivery issues.
The consistency of the carrier gas makes the process of control at the furnace achievable. Although the carrier gas can vary between nitrogen methanol and endothermic gas, the desired theoretical makeup can typically be found as 40 percent hydrogen, 40 percent nitrogen, and 20 percent carbon monoxide. Maintaining these percentages will result in a carburizing atmosphere that is conducive to best carburizing practices.
Endothermic atmospheres are produced by mixing air and natural gas (methane) or air and propane, heating them to elevated temperatures and reacting them in the presence of a catalyst to form a specific percentage of carbon monoxide, nitrogen, and hydrogen. The reaction is shown using air with an approximate composition of 80 percent nitrogen and 20 percent oxygen.
2CH4 + 4N2 + O2 → 2CO + 4H2 + 4N2
Small amounts of carbon dioxide and methane are also produced or left unreacted. Typical endothermic gas created from natural gas and air consists of approximately:
40% nitrogen — N2
40% hydrogen — H2
18.8% to 20.5% carbon monoxide — CO
0.25% – 0.50% carbon dioxide — CO2
0.50% or less methane — CH4
Nondispersive infrared analyzer (NDIR) systems are invaluable when trying to troubleshoot these issues. An analyzer will typically measure CO, CO2 and CH4. As mentioned earlier, if we know that 20 percent CO is being generated, we can cross-check the air/gas ratio and sticking flow meters, or determine that an adjustment of the air and/or gas ratio is required. The measurement for indication of sooted or nickel-depleted catalyst also can be achieved using an analyzer. If the indicated measurement of CH4 is higher than 0.5 percent, a burnout of the catalyst is required, using the manufacturer’s required procedures. If, after a burnout, the CH4 level is still high or increases within a few days, the catalyst may need to be replaced altogether.
NDIR analyzers are also used to derive carbon in atmosphere furnaces. The combination of the temperature, CO, CO2, and CH4 provide enough data to calculate the carbon potential in the furnace. This technology allows for minor adjustments to the in-situ carbon probe controller calculation to minimize out-of-control carbon situations with the ultimate goal of producing quality parts without rework or — in the worst-case scenario — scrap.
NDIR Theory of Operation
The cells operate according to the Non-dispersive Infrared (NDIR) alternating light to generate frequency. The measurement principle is based on the band-pass filters for each specific absorption range of the gases being measured. Measurement of carbon monoxide, carbon dioxide, and methane gas is highly selective.
Atmosphere Gas Composition
With all enriching gas and dilution air valves manually closed on the furnace just flowing endothermic gas, your gas composition should look similar to the endothermic gas being read directly from the endothermic generator.
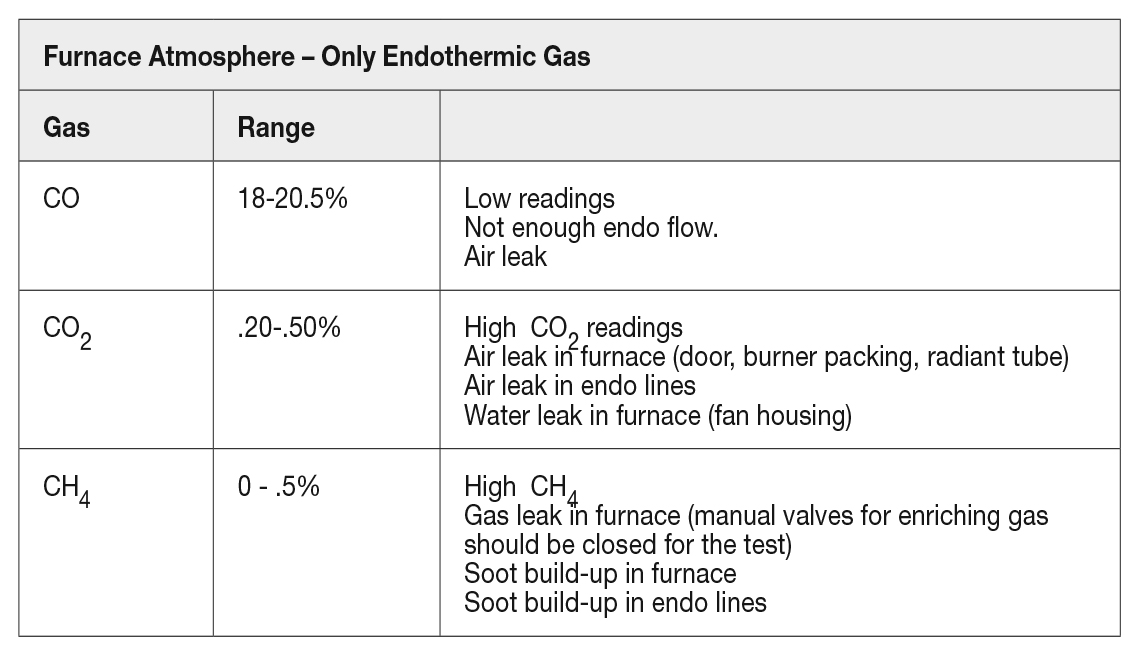
The furnace atmosphere expected from a prepared atmosphere only can be seen in Table 1.
When issues arise with the atmosphere, a good test and evaluation for potential issues can be identified by using straight prepared atmosphere and evaluating the composition of the gas as described.
If the endothermic generator gas or the nitrogen methanol system are not producing the proper gas, these values will be different (variations in straight nitrogen methanol gas will vary based on furnace temperature). Always start with your prepared atmosphere first and make sure your carrier gas is consistent for what you are expecting at the furnace.
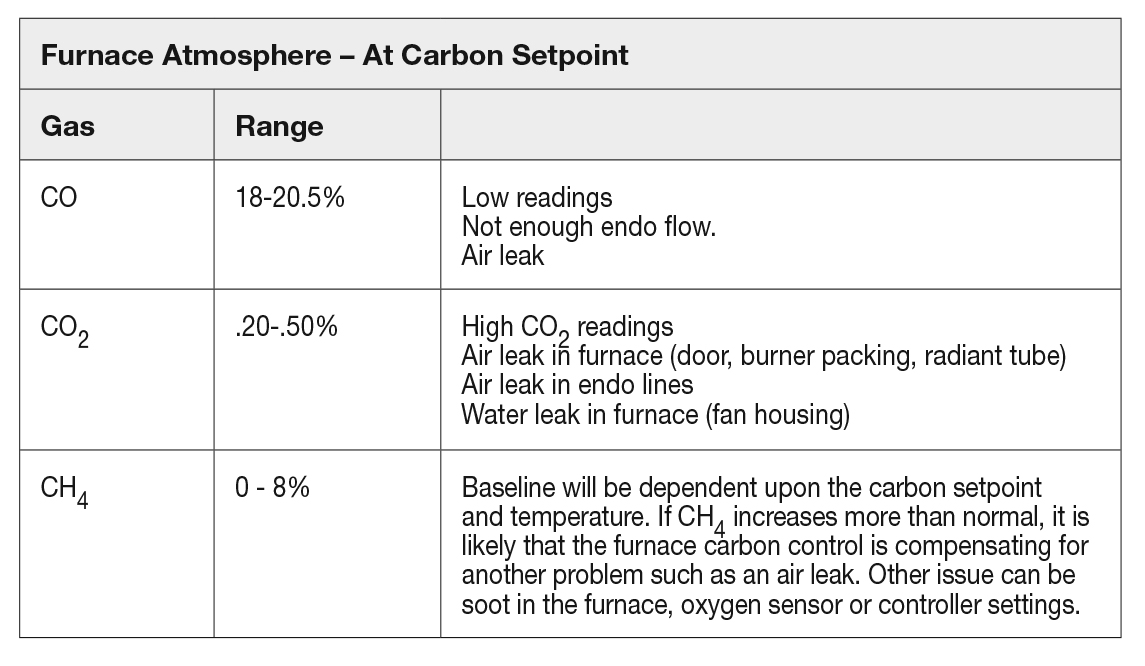
To get a handle on the furnace atmosphere composition, one should have a baseline for the gases that produce good metallurgical results at specific atmosphere settings. Since each furnace will end up being slightly different, it is recommended that each furnace be evaluated. The furnace will have varied readings based on burnout schedule and carbon potential setpoint being used. The variations typically are caused by “seasoning” of the furnace. Any change in the ability to control the furnace around a desired setpoint can be caused from any of the issues described in Table 2.






























