Microstructure optimization of steel for improved performance requires detailed knowledge of the thermal response of the materials. When designing heat treatments of new materials in-situ information about, for example, phase transformation, is pivotal. In this work, the use of two complimentary techniques for in-situ monitoring of thermal behavior of carbon and nitrogen containing steels, i.e., dilatometry and combined calorimetry and thermogravimetry is presented. To illustrate the importance of in-situ techniques for understanding materials behavior, three different examples are presented. The examples presented are: 1) high carbon martensitic (stainless) steel overspray powders for additive manufacturing, 2) high temperature solution nitriding and the role of nitrogen on microstructure in conventional martensitic stainless steel, and 3) additively manufactured 17-4PH martensitic stainless steel and the role of nitrogen. It shows how in-situ techniques can be applied to record the heat-treatment response of these generically different special materials. It is also emphasized how nitrogen can be actively used in steels, which can pave the way for more widespread use of nitrogen containing steels. In addition to in-situ techniques, light optical microscopy and X-ray diffraction analysis are used for characterization.
1 Introduction
Various in-situ methods can track real-time heat-treatment response in bulk materials. This can aid material engineers in designing the most effective heat-treatment procedures and processing methods. In-situ methods such as thermogravimetry (TG), differential thermal analysis (DTA), and dilatometry, offer capabilities to examine heat-treatment behavior in real time, giving insight into the thermochemical mechanisms and the thermal behavior of steel [1].
This contribution specifically addresses different martensitic (stainless) steel where in-situ control during heat treatment and detailed understanding of their microstructure can pave the way for new applications. The examples presented are 1) heat-treatment behavior of different classes of high-carbon martensitic (stainless) steel powder for additive manufacturing application, i.e. stainless 440C, cold-work D2, hot work H13, and high-speed steel (HSS) T15, 2) nitrogen alloying of wrought martensitic stainless steel AISI 420, and 3) heat-treatment response of additively manufactured and conventional precipitation hardening maraging stainless steel 17-4PH. This work is not an in-depth treatise of these individual topics but is intended to show the importance of in-situ techniques for development of new materials (solutions). The individual topics are briefly introduced in the following.
1.1 High carbon steel powders for additive manufacturing
Additive manufacturing (AM) is gaining popularity and experiencing rapid technological advancements. Recently, there has been a significant focus on the application of high-carbon steels [1]. AM methods, such as laser powder bed fusion (LPBF), binder jetting (BJ), and spray forming offer several benefits, including the attainment of uniform microstructures that offer superior mechanical properties compared to conventionally manufactured metals. Currently, several specialized high-carbon powders are commercially available for AM. For a successful adoption of high carbon steels for AM, more knowledge on the fundamental thermal behavior related to the processing is needed. To this end, powders and in-situ techniques are an excellent foundation for investigating heat-treating characteristics.
440C stainless steel (440C) is a high-carbon martensitic stainless steel known for its high hardness, wear resistance, and moderate corrosion resistance. According to Bang et al. [2], LPBF addresses the limited industrial applications caused by 440C’s high hardness and low workability, while also improving overall mechanical properties such as ultimate tensile strength (UTS) and yield strength (YS) [4,5].
D2 cold-work steel (D2) is distinguished by its superior hardness, high strength, and excellent wear resistance, making it widely used in industrial applications like cutting and punching tools, as well as dies. This material is well-suited for AM techniques, particularly direct energy deposition (DED) [5]. H13 hot-work tool steel (H13), typically used in a quenched and tempered state, features a martensitic matrix with dispersed fine secondary carbides. Known for its high hardness and fracture toughness, H13 also offers excellent wear and erosion resistance, along with relatively high resistance to thermal shock and thermal fatigue. As noted by Park et al. [5], metal deposited via the DED process exhibits different properties compared to wrought metal due to the rapid solidification rate and the high thermal gradient between the deposited metal and the substrate. The microstructure of deposited D2 and H13 has been shown to be highly uniform, with the hardness of deposited D2 comparable to conventional martensitic high-carbon stainless steel, and the hardness of deposited H13 exceeding that of wrought H13.
T15 high-speed steel (T15) is well known for its high hardness and excellent wear resistance at elevated temperatures, attributed to its significant carbon and tungsten content, making it an ideal material for cutting tools, drills, blades, and knives. According to Zhang et al. the spray-formed T15 steel exhibits higher hardness, significantly enhancing its overall mechanical properties [6]. This increase in hardness is a crucial factor contributing to the superior mechanical performance of T15 steel in various applications [8-11].
1.2 Nitrogen alloying to martensitic stainless steel, high temperature solution nitriding
Adding nitrogen to stainless steel may be beneficial to the bulk properties of the material, such as increased surface hardness and optimized microstructures that retain the corrosion resistance. Nitrogen can be added in the liquid phase during fabrication of the steel, but this is usually an expensive and cumbersome process as it requires high N2 pressures and there is the risk of formation of nitrides during cooling and subsequent processing. An alternative is “High Temperature Solution Nitriding” (HTSN), where nitrogen is introduced from the gas phase to the solid state — a process which is highly analogous to classical carburizing of non-stainless steels. Nitrogen is typically added to stainless steels at temperatures ranging from 1,050-1,150°C and using pressures of N2 ranging from 0.1 to 3 bar [11,12]. The process entails gas quenching in N2 to suppress formation of chromium nitrides. The entire process is carried out in a clean environment and will provide improved corrosion resistance, higher hardness, and wear resistance and enhanced fatigue performance. The process is particularly interesting for martensitic stainless steels but can also be applied to austenitic and duplex stainless steels. Depending on a wide variety of process conditions, some being the alloy content, nitrogen pressure, and the subsequent cooling rate, the resulting final microstructure can vary quite vastly. Additionally, the total amount of nitrogen taken up by the material will also affect the final microstructure, for example the phases developed (viz. martensite) and the morphology of martensite [13]. Adding nitrogen through HTSN to martensitic steels can come at a cost. Since nitrogen itself is a strong austenite stabilizer, this can produce higher amounts of retained austenite following quenching, which many times are undesirable [14,15].
The complexity of diverse alloying elements and process conditions means that process optimization can be tedious and require many iterations. The interplay between retained austenite and solution hardening associated with martensitic stainless steels are considered through the analysis of sample microstructures of the investigated materials. In-situ methods offer the benefit of completing lab scale iterations, achieving fast results in a clean and controlled environment. As mentioned previously, the HTSN treatment can produce “case-hardening-like” results. The discussed in-situ techniques can be applied to other traditional gaseous case hardening processes such as carburizing of steels, where similar uptake behavior is present.
1.3 17-4 Precipitation hardening steel
Precipitation hardening (PH) steels, such as 17-4PH, are martensitic stainless steels that harden through forming precipitates during an aging treatment. The standard heat treatment for 17-4PH for peak hardness is the 900H treatment, involving a solution treatment about 1,040°C, quench, then age at about 480°C (≈900°F) for one hour [16]. 17-4PH primarily forms Cu precipitates in the martensite phase, so when the quenching fails to complete the martensite transformation, retained austenite will limit the hardness of the final material [17].
In AM, LPBF 17-4PH can have a primarily austenitic structure due to N2 gas-atomized powders and N2 cover gas used during processing. In addition to the austenite stabilizing effects of nitrogen, LPBF 17-4PH tends to have a cellular or dendritic structure associated with micro-segregation of the alloying elements. This further suppresses the martensite transformation, leading to a primarily austenitic microstructure after heat treatment.
Like other AM metals, the unique composition and microstructure of LPBF 17-4PH requires a modified heat treatment to achieve the desired material properties. Other studies have found success in lowering the solutionizing temperature [17], extending the aging time [18], or incorporating sub-zero Celsius treatments [19].
2 Experimental procedures
2.1 Materials
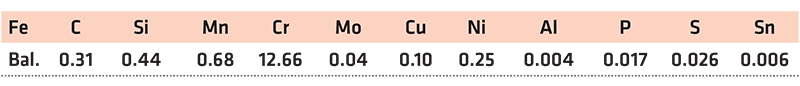
The high-carbon steel powders investigated were delivered from Asgaard Metals and were overspray from spray forming of ingots. The compositions as provided by the supplier are given in Table 1.

Rods of AISI 420 stainless steel with the diameter of 12.7 mm (1/2”) from McMaster-Carr were used. The material was received in the hot rolled and cold annealed state. The composition as provided by the supplier is listed in Table 2. The rod was cut using a precision saw into a 5 mm thick disk to be used for thermogravimetric measurements.
Wrought 17-4PH samples, 5 mm (3/16”) diameter rods supplied from McMaster-Carr in a cold-worked, solutionized and unaged state were used. LPBF samples were produced by GKN using their nitrogen gas-atomized ANCOR AM 17-4PH powder. The composition of 17-4 rods and powder is given in Table 3. The LPBF set was made using standard parameters with a N2 cover gas. For dilatometry, rods of 10 mm length and 5.5 mm in diameter were machined with the long axis parallel to the building direction. The rods were then cut into bars of 10 mm in length.

2.2 Thermal gravimetry (TG) and differential thermal analysis (DTA)
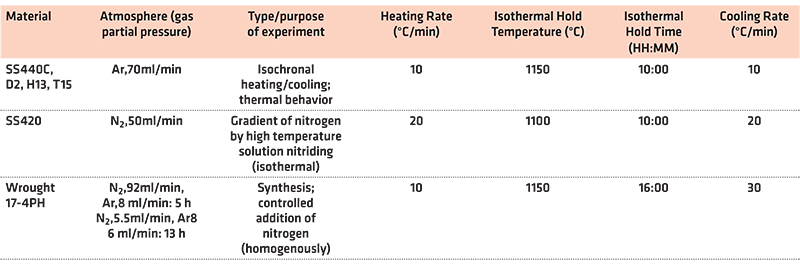
Both TG and DTA results were obtained using the Netzsch STA 449 F3 thermal analyzer. For TG analysis of AISI 420, a 2.9 g round bottom alumina crucible was used. The sample was positioned with one flat side facing upwards. This crucible was measured as having a 15.75 mm internal diameter. Samples of high carbon stainless steel powders were poured into flat-bottom alumina crucibles with an internal diameter of 6.00 mm and an internal depth of 3.75 mm. The flat bottom crucibles were filled to about 3/4 of their maximum capacity. Immediately following the purge cycle of inert gas, these crucible samples were placed onto the sample holder in the furnace compartment. For each thermal analysis, the furnace chamber and balance system were put under vacuum and then backfilled with argon or nitrogen. The furnace chamber was then heated according to the specific temperature program set. The main parameters set can be seen in Table 4. The nature of the performed experiments is indicated in the table.
2.3 Dilatometry
Dilatometer tests were run on the TA Instruments DIL805 following the peak hardening treatment for 17-4PH. Under vacuum, 1,037°C for three hours, followed by a quench to room temperature in He gas at a rate of minus-33.6 K/s. It is then aged at 482°C for one hour under vacuum and then quenched.
2.4 Metallography
Samples were cut using a Buehler IsoMet High Speed Pro precision saw to obtain cross sections for examination. Blades were chosen to fit the specific material being cut. Each sample was mounted in a Buehler Simplimet 4000 mounting system, using Buehler PhenoCure compression mounting compound. All mounted samples were polished on the Buehler AutoMet 250 according to Buehler’s standard four-step method for stainless and maraging steel polishing. Samples were then etched using Kalling’s reagent, which consists of 33% hydrochloric acid, 33% ethanol, 33% deionized water, and 1% cupric chloride dihydrate. The samples were swabbed for approximately five seconds, terminated with deionized water, and cleaned using ethanol. Micrographs were obtained using a Nikon Epiphot 200 Microscope. Vickers hardness tests were completed using the Wilson VH3300 automated hardness tester using a load of 0.5 kg, and a 10 second dwell time.
2.5 X-Ray diffraction (XRD)
XRD analysis using Cr K-α radiation with wavelength 2.28976 A° using a PANalytical Empryrean X-ray diffractometer. For the high-carbon steel powder, XRD analysis was done using Cu K-α radiation from 30-125° 2θ angle. Step size 0.03 degree and step time 15 seconds. The results were analyzed using Origin and the recorded line profile was smoothed by using Savitzky-Golay method.
3 Results and discussion
3.1 High carbon martensitic steel powders
The overspray high carbon steel powders are unique in the way they are a byproduct from spraying of steel ingots, i.e., they are rapidly solidified and cooled. Hence, the microstructure is not necessarily conventional, which emphasizes the importance of in-situ tracking of their thermal behavior.
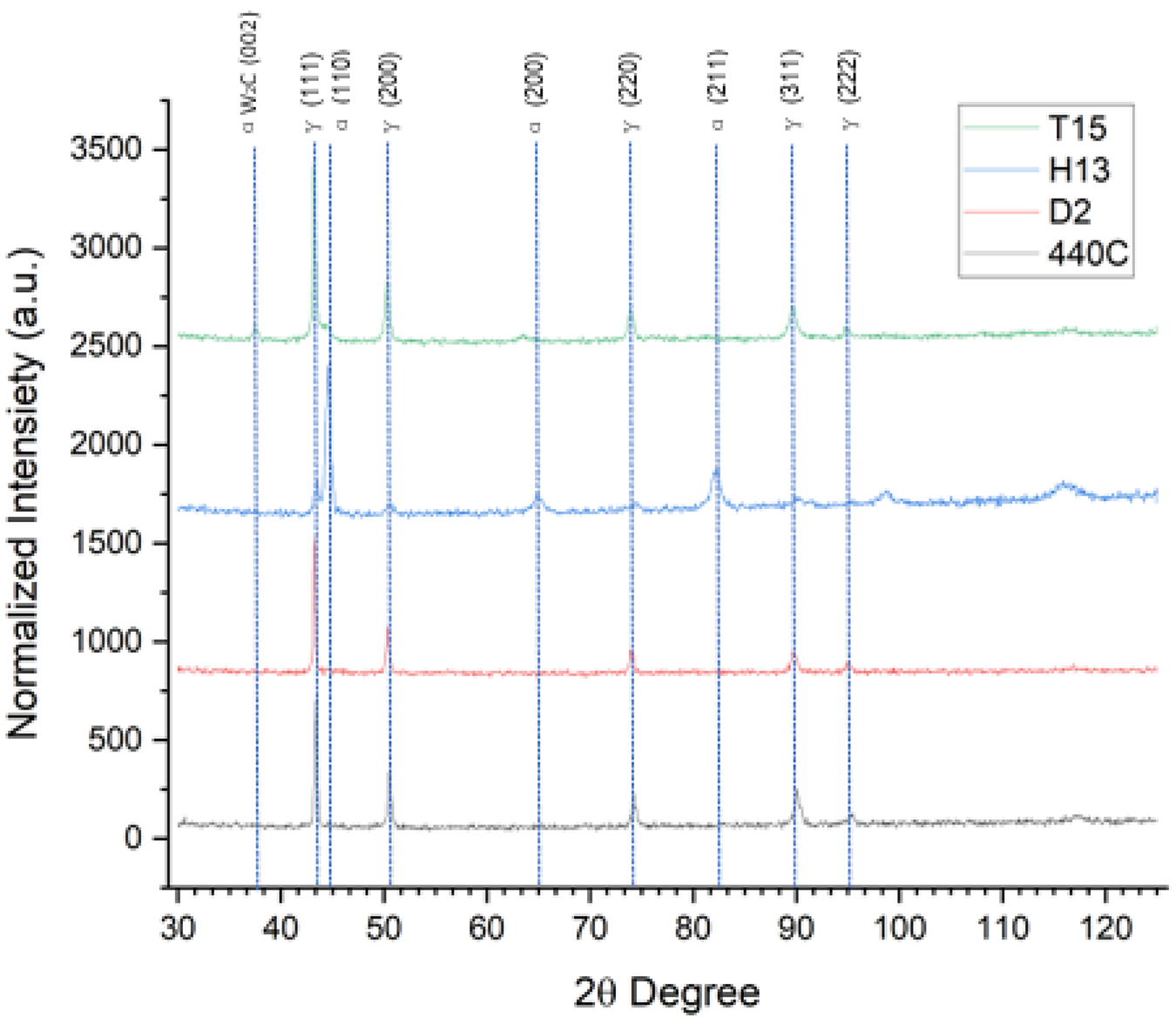
XRD analysis of the as-delivered powders are given in Figure 1. The materials 440C and D2 are fully austenitic, which can be attributed to fast cooling (inherent to the process) combined with a high interstitial content. T15 is also predominantly austenitic with a minute fraction of ferrite/martensite; minor peaks of W2C type carbides can also be observed. H13 is predominantly ferritic/martensitic with a minor fraction of (retained) austenite.
DTA during isochronal heating can record phase transformations or reactions associated with release or uptake of heat (calorimetry). Upon heating, the powders 440C, D2, and T15 undergo an exothermic reaction in the temperature range of 620-750°C, which can be attributed to (partial) decomposition of austenite, presumably via eutectoid decomposition, i.e., alloy pearlite. This transformation is most pronounced for 440C and least pronounced for T15, which correlates with the amount of retained austenite in the initial condition. A second peak, occurring between 800-900°C for all materials, is attributed to the formation of austenite (Ac1). Examination of the DTA signal (Figure 2) indicates that the austenitization start temperature for SS440C is approximately 820°C, with complete transformation at 850°C. For D2, austenitization begins around 820°C and completes at approximately 860°C. In the case of H13, austenitization starts at around 850°C and concludes at about 900°C. For T15, austenitization commences at about 810°C and is complete at approximately 875°C.
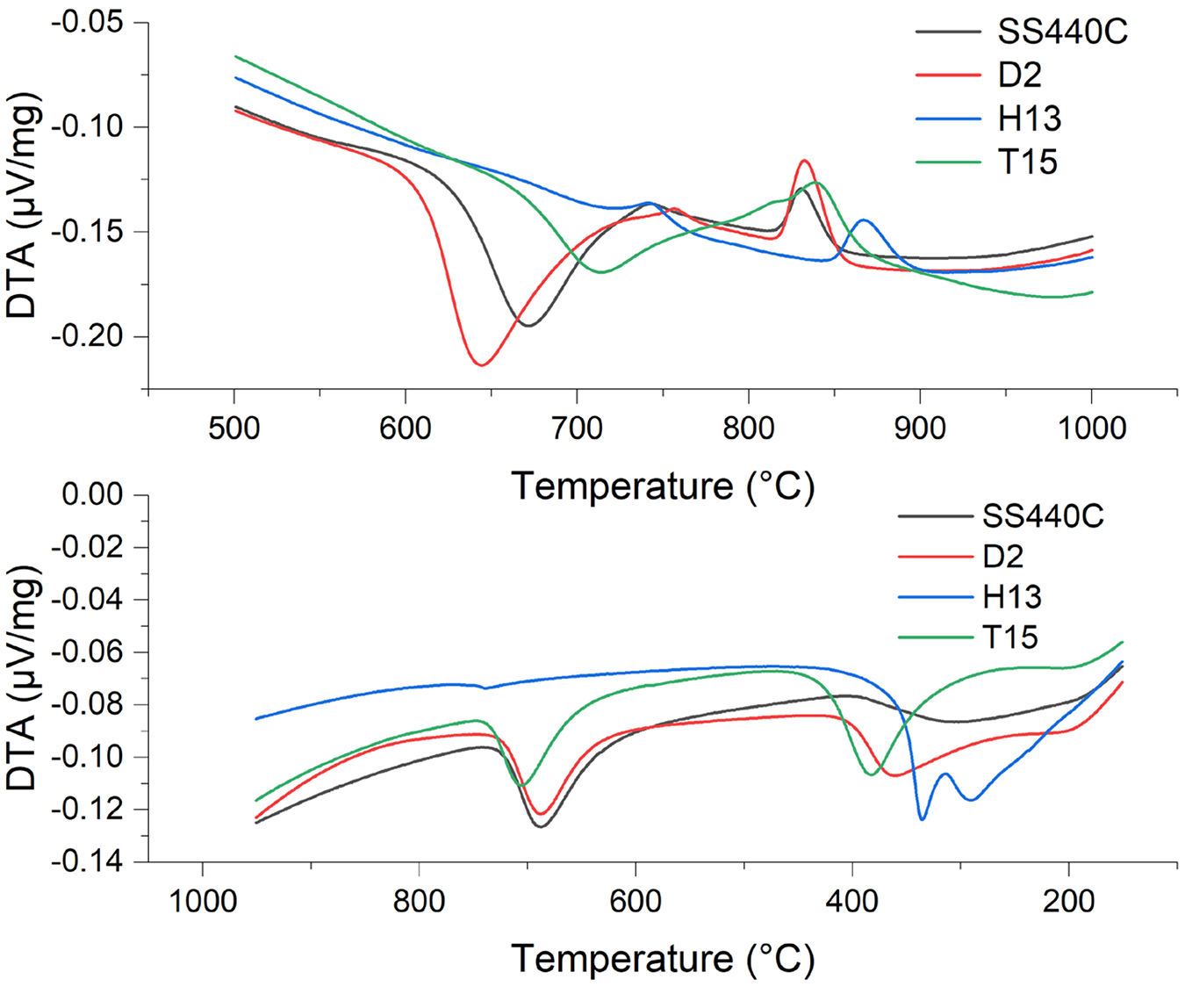
The calorimetry signals from cooling at a rate of 10 K/min are given in (Figure 2). The exothermic peak for the carbon rich SS440C, D2, and T15 indicates the eutectoid transformation of austenite into alloy pearlite occurring during cooling at about 750-650°C, analogous to the transformation taking place during heating. The second peak in the DTA signal (for all alloys) during cooling indicates formation of bainite at about 375°C followed by martensite formation. This behavior is consistent with CCT diagrams of the conventional wrought materials (not shown herein).
3.2 HTSN of AISI 420
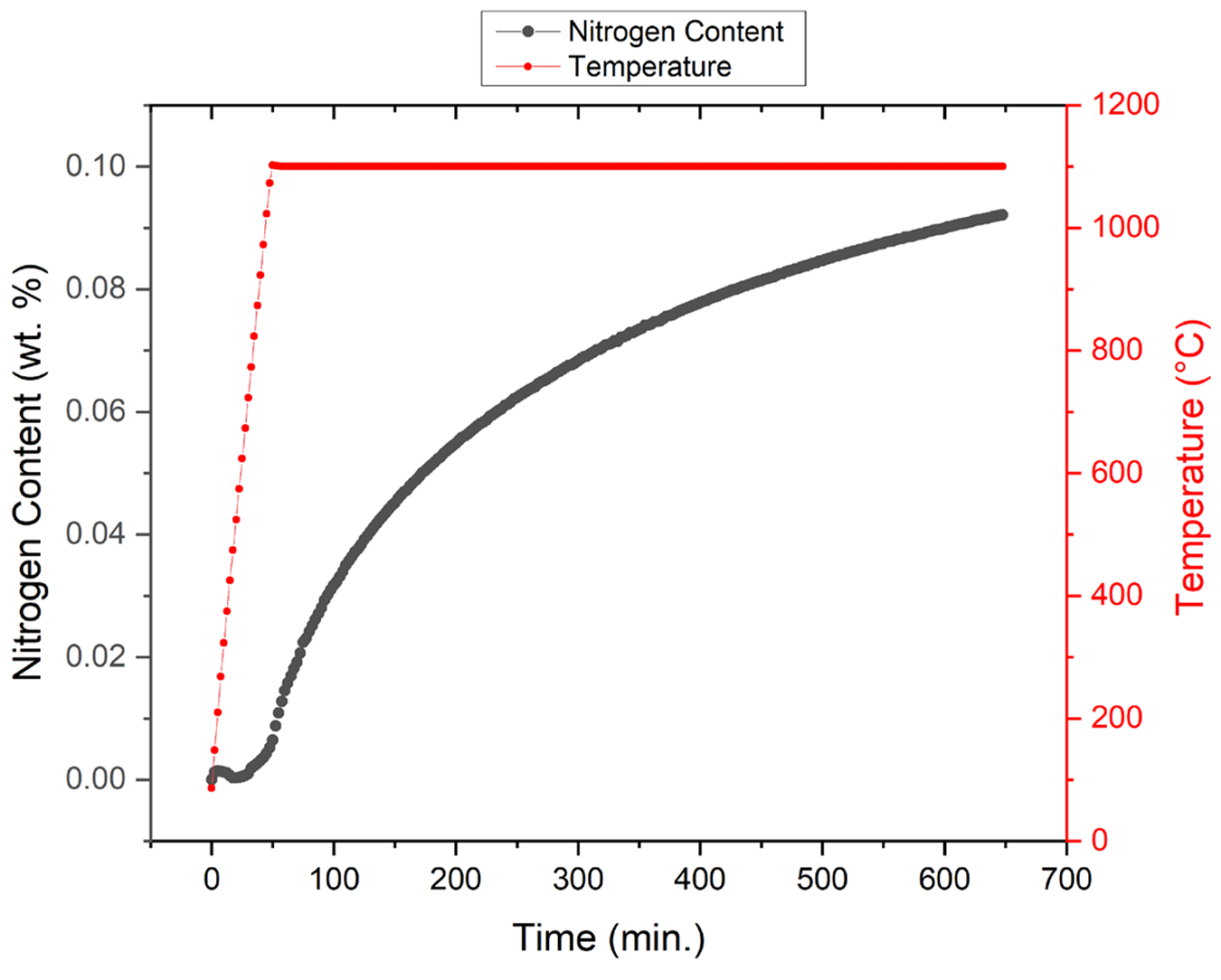
To assess the role of nitrogen in martensitic stainless steels, high-temperature solution nitriding can be used, which will result in a graded structure provided the sample is not through-nitrided. Herein, the widely used AISI 420 is selected to illustrate the impact of nitrogen on the microstructure. Please note the solution nitriding temperature of 1,100°C coincides with the conventional temperature for austenitization of this material. The applied cooling rate from the nitriding temperature is relatively slow, but here it is merely to demonstrate the effect of nitrogen rather than to present an optimized process. Figure 3 depicts the results obtained for in-situ gaseous nitriding through TG analysis. This graph represents the temperature and mass uptake of nitrogen in the sample. As can be observed in the figure, the total sample uptake prior to cooling comes to approximately 0.09 wt.% nitrogen. The flux of nitrogen follows directly from the in-situ recorded uptake of nitrogen during nitriding, when considering the specimen’s surface area. The overall nitriding kinetics seems to follow a parabolic growth law indicating diffusion-controlled growth rather than growth governed by surface kinetics. It should be noted that the weight percentage of nitrogen is measured in the entire sample, with a diffusion gradient of interstitial nitrogen moving from high concentration at the edge to a lower concentration at the center according to Fick’s second law of diffusion [20]. Hence, the surface region of the sample has a significantly higher nitrogen concentration than the overall 0.09 wt%, whereas the core has essentially no nitrogen.
Micrographs at different magnifications were obtained from two regions of the 420 stainless steel, adjacent to the surface where the nitrogen concentration is highest and at the center of the sample. Figure 4a represents the surface region of the 420 stainless steel disk and Figure 4b represents the center of the disk, which is essentially without nitrogen, but contains carbon.
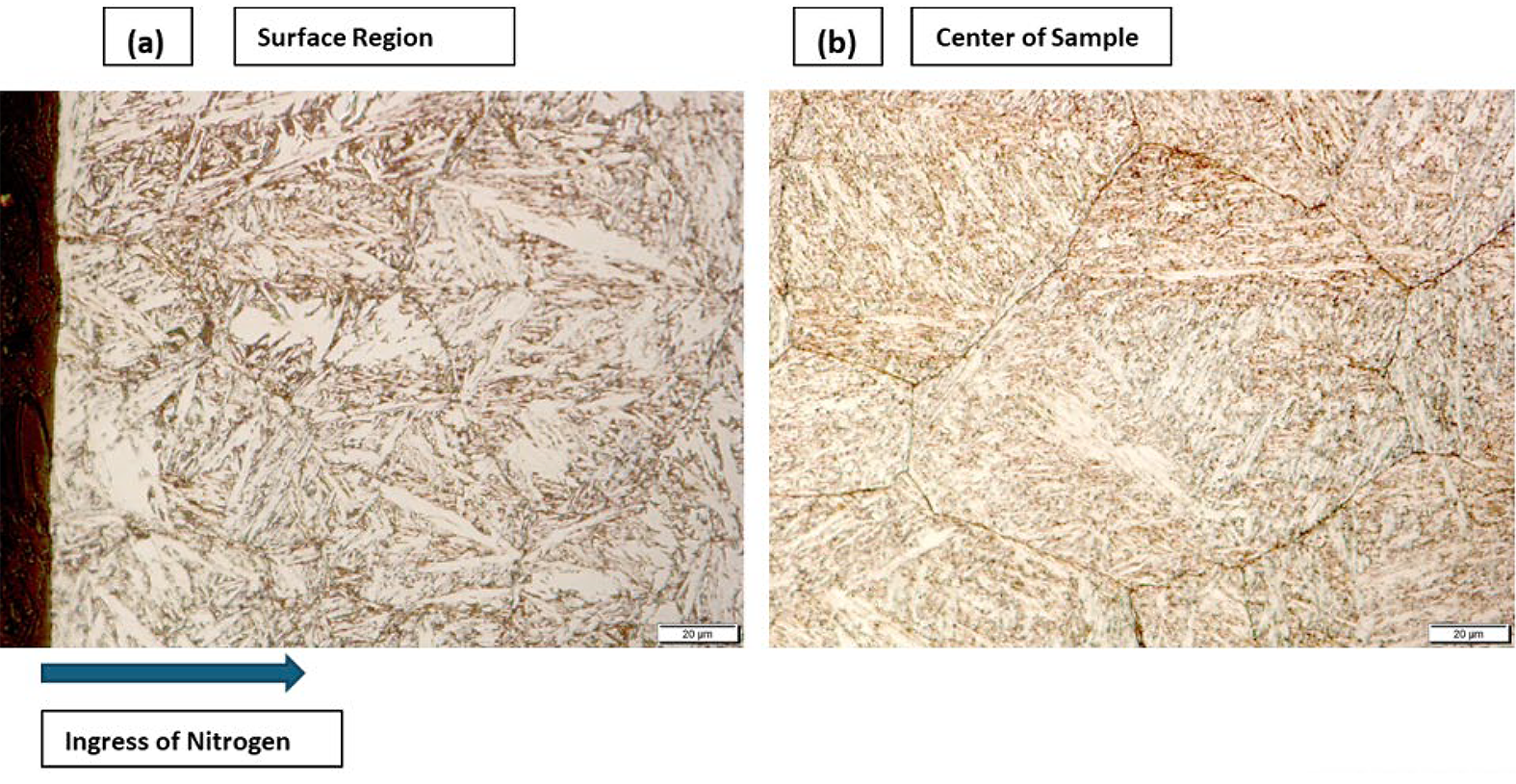
As shown in the micrographs (Figure 4), there is a noticeable difference in the grain structure and phases within the sample. Due to the transient nature of this gaseous process, it can be inferred there is a higher concentration of nitrogen near the surface of the sample, which was in contact with the gaseous atmosphere. In Figure 4a, the grain structure can be seen to be finer, albeit with larger martensitic plates. These plates can be identified by their white color. Figure 5 shows a closer view of this morphology, which appears to be lenticular or thin-plate martensite, and it has a characteristic zigzag pattern. This type of morphology is related to an increased nitrogen content [21,22]. It could be inferred that the dark portions indicate the presence of some form of nitride, likely chromium nitrides, which decorate the outer grain boundaries.
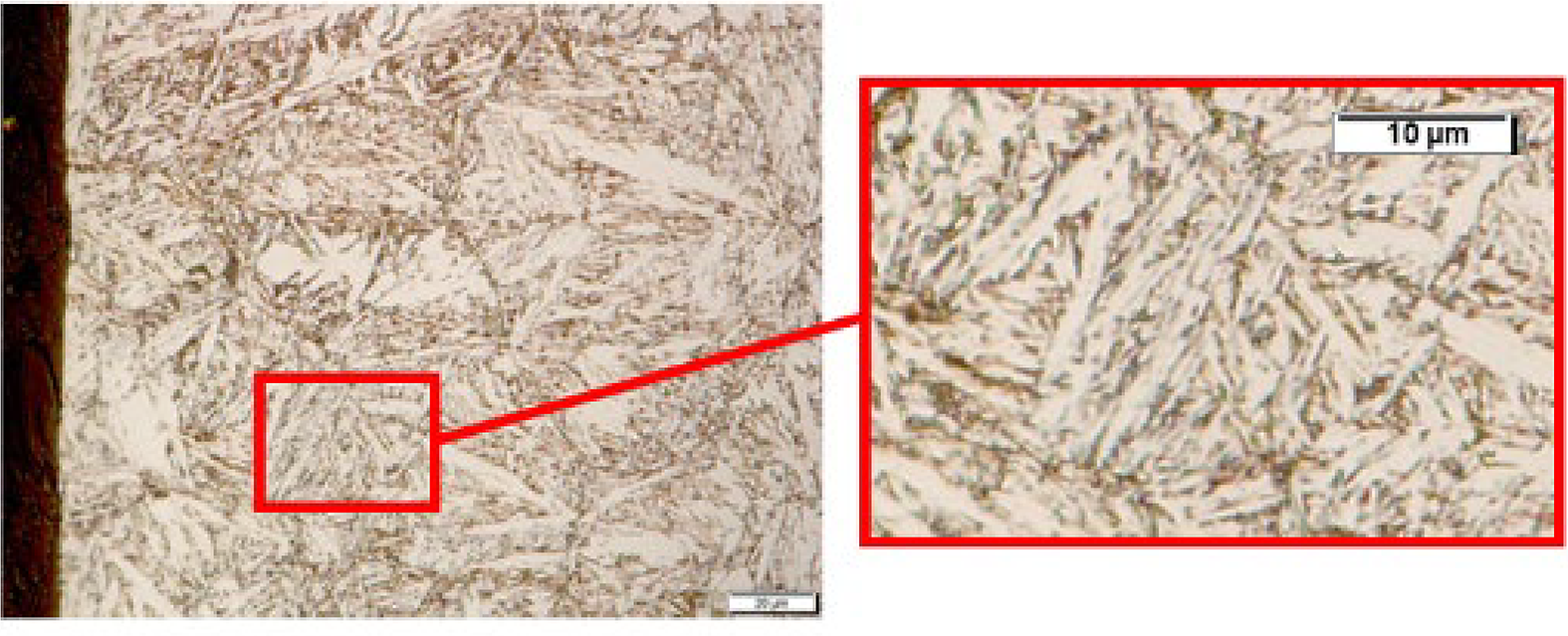
Figure 4b shows much larger grains than can be seen in Figure 4a. This feature can be, in part, due to the absence of grain pinning in this region. Initial formation of nitrides will occur in the surface region during heating to the nitriding temperature (1,100°C); at the nitriding temperature the nitrides will dissolve. The presence of chromium nitrides can pin grain growth near the surface where the nitrogen content is high [21]. The different composition of interstitial elements between case and core can also play a role for grain growth of the austenite. The micrograph indicates the presence of lath martensite. Nitride formation should not take place, so the grain boundary decorations observed are likely carbides forming during cooling. The presence of nitrogen in this sample demonstrates an interesting relationship between solution hardening and retained austenite, where the former serves to harden the material and the latter should soften the material when compared with martensite [20]. The higher the interstitial content in martensite, the greater the hardness. However, the higher the content of interstitials dissolved in the austenite, the lower the Ms temperature, and therefore, more retained austenite is present. The greater retained austenite fraction, the softer the material becomes. Here it should be mentioned that nitrogen and carbon have a significant solid solution strengthening effect in austenite; this effect is much more pronounced in stainless steels than in non-stainless steel. Another factor which can contribute to this balancing relationship can be shifting of the martensitic start (Ms) temperature based upon the size of austenitic grains prior to martensitic transformation. A smaller grain size will lead to a lower Ms temperature; this effect works in conjunction with the nitrogen content. In terms of martensite morphology higher nitrogen contents (lower Ms temperature), will result in a more plate-like morphology. Low nitrogen contents will be associated with a lath type martensite in this type of material [23]. Examining Figure 4b, the micrograph indicates the presence of lath martensite [21,22] representative of a low nitrogen content.
Upon measuring the hardness of the HTSN treated 420 stainless steel sample, it is apparent the hardness is relatively unchanged over the cross-sectional distance through the sample. Figure 6 shows reasonably stable hardness readings with the average hardness measuring at approximately 673 HV. This value is in the upper range of what is achievable based upon the carbon content in the sample. There is a slight dip toward the sample’s edge that may be attributed to increased nitrogen content and higher fraction of retained austenite (cf. micrographs above). Sub-zero treatment could be applied to minimize the amount of retained austenite.
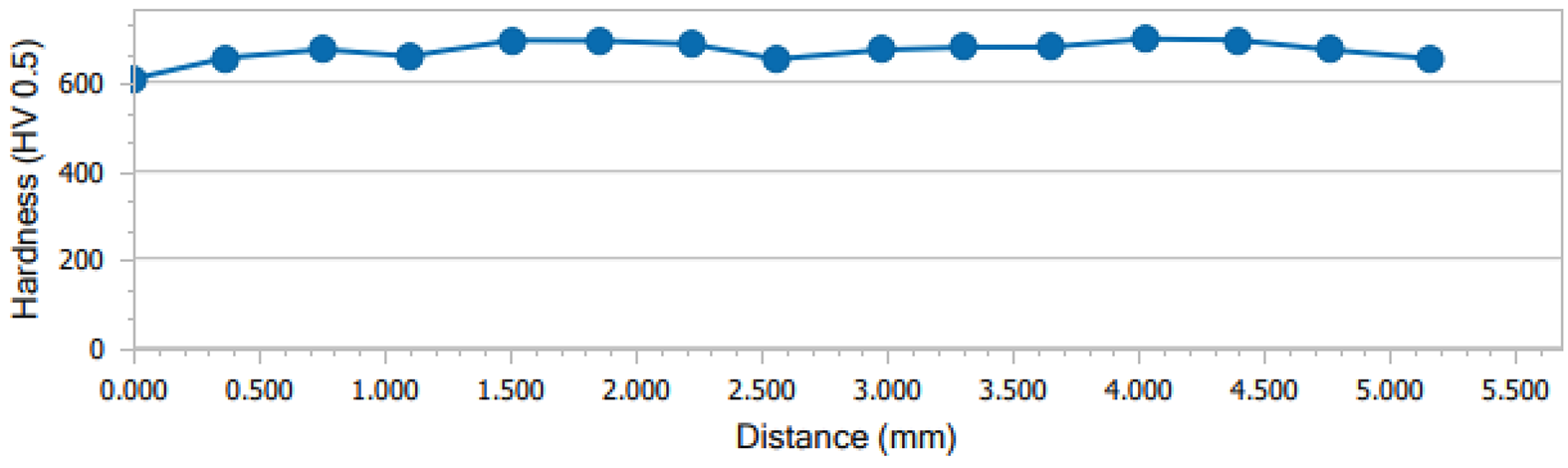
When compared with the hardness of untreated 420 stainless steel, there is quite a significant hardness increase. The as-received hardness of 420 stainless steel in soft-annealed condition is approximately 200 HV. The hardness obtained from the HTSN treatment is quite similar to the hardness yielded by a traditional oil-quenched sample (approximately 610-740 HV) [23]. Using nitrogen, this maximum achievable hardness range can be extended.
For in-situ determination of Ms temperature, dilatometry can be applied but requires uniform nitrogen over the full width of the tested sample. By using the in-situ thermal analysis, both HTSN processes as well as other traditional case hardening processes, could be refined. The unique feature of in-situ nitriding, carburizing, etc., is the ability to monitor the uptake kinetics as a function of time and/or temperature. The ability to carry out lab scale treatments in a controlled environment can save resources and allow for a more calculated and iterative approach to optimizing treatment.
3.3 Precipitation hardened stainless steel
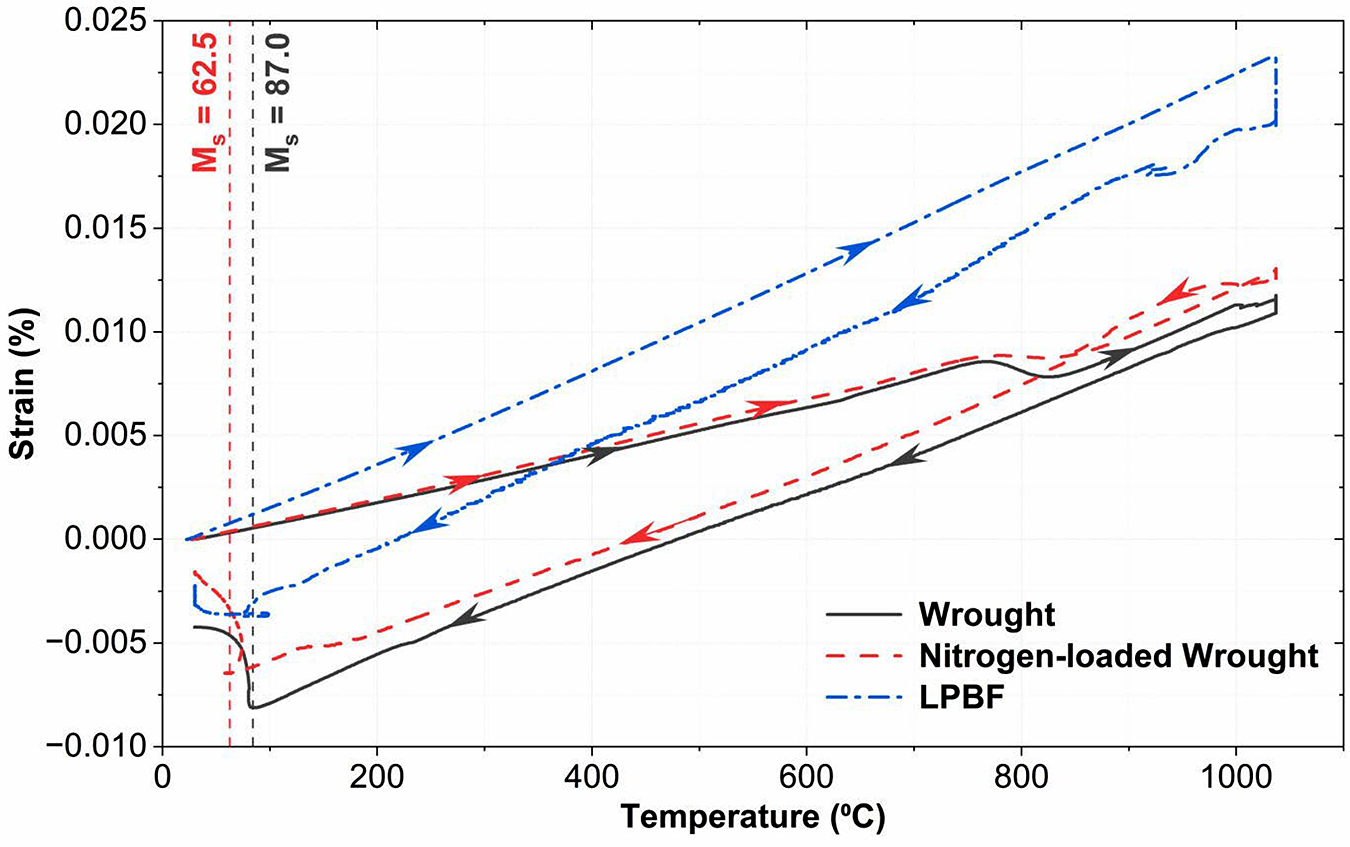
The wrought 17-4PH had a starting nitrogen content of 0.027 ± 0.0002 wt% N, the nitrogen-loaded wrought had 0.16 ± 0.004 wt% N, and LPBF had 0.13 ± 0.006 wt% N. Both wrought samples show similar slopes upon heating in the dilatometry curve (Figure 7) until a change in slope around 775°C, representing a phase transition. After quenching, both samples follow a new slope until a new change in slope at low temperatures, marking the martensite transformation. The point where this sharp transition begins can be labeled as the Ms temperature for that curve. The dilatometry curves for the wrought samples show that both started with a martensitic structure, transformed into austenite, as represented by the steeper slope, and then transformed back into martensite. The LPBF curve maintains the steeper slope before and after quenching and does not show any significant transformation curve. This suggests that the LPBF began with a primarily austenitic structure and did not reach its Ms temperature upon quenching.
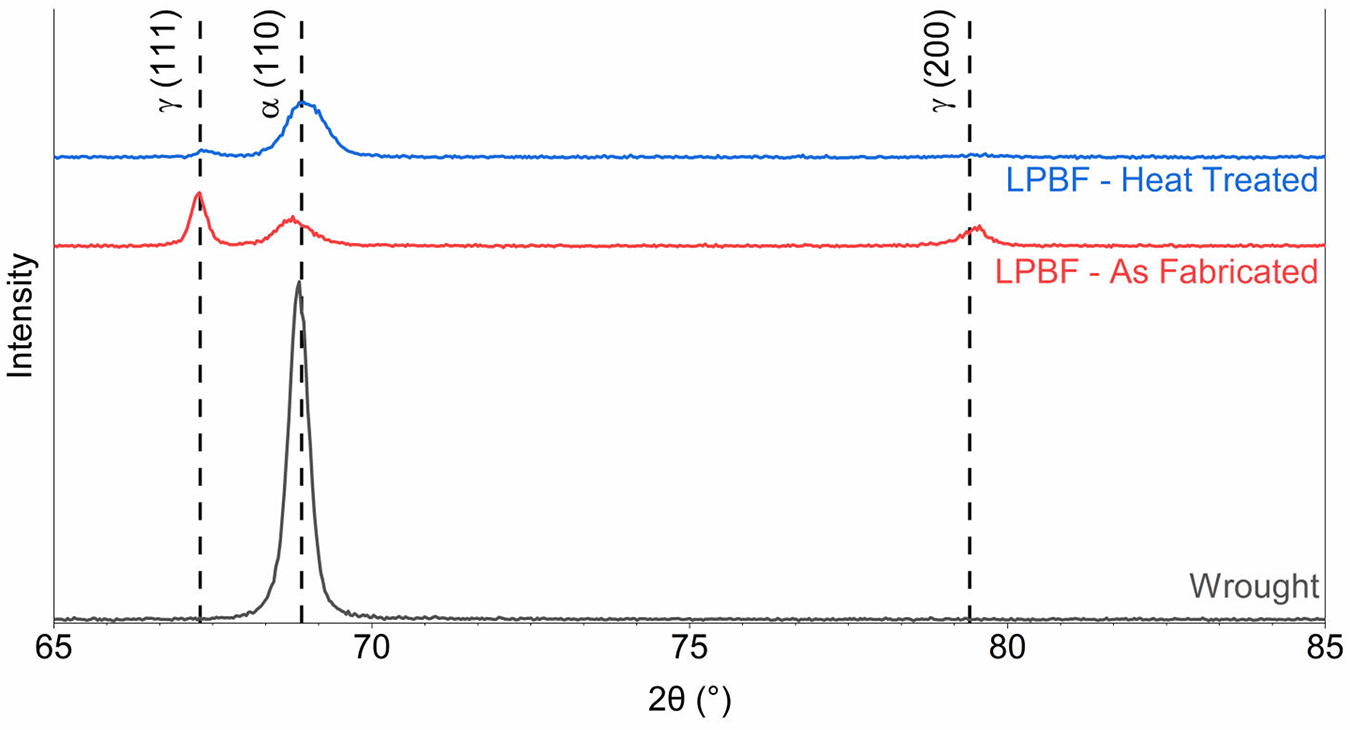
The primarily austenitic structure is also reflected in the XRD results, plotted in Figure 8. Before heat treatment, LPBF displays intense austenite (γ) peaks and small ferrite (α) peaks. After heat treatment, the ferrite peaks become more prominent, but the austenite peaks remain, suggesting a partial martensite transformation. In comparison, the wrought 17-4PH has a completely martensite microstructure, as reflected by the intense ferrite peak.
Despite having a lower nitrogen content, the dilatometry curves show that LPBF remains austenitic for the entire heat-treatment cycle while the nitrogen-enriched wrought starts and ends martensitic. This may be due to the anisotropic and dendritic/cellular structure characteristic of LPBF further suppressing the martensite transformation [24].
3.4 General discussion
As various AM methods will be applied to these powders, investigating the thermal response of the overspray powder is crucial for understanding the relationship between microstructure and properties. This investigation can provide insights into how different AM processes influence the microstructure and, consequently, the mechanical properties of the material. Furthermore, optimizing the microstructure and tailoring the properties can enable the customization of these materials for specific applications, enhancing their performance and suitability for a wide range of industrial uses.
Nitrogen can be picked up in both the feedstock powder and from the cover gas in part fabrication. Most conventional stainless steels have a low nitrogen content, so the impact of nitrogen in AM stainless steels must be addressed through in-situ techniques to develop a modified heat treatment.
Nitrogen has shown promise in being a viable alternative to interstitial carbon in gaseous treatment of steels. It demonstrates similar “case hardening” properties in steels and can be especially beneficial in stainless steels. However, using too much nitrogen can come at a cost, since austenite stabilization may not be desired. It is for this reason that optimization of microstructure is necessary, where in-situ techniques may be implemented. By using controlled heat-treatment tracking methods, lab scale iterations can be completed, assisting in the specific tailoring of microstructures. This information can be used and adopted on wider scale industrial processes to increase the properties and performance of materials.
4 Conclusion
Three different martensitic and interstitially alloyed materials systems were investigated using the in-situ techniques: dilatometry, calorimetry, and thermogravimetry. These analytical techniques provide critical insights into the structural and thermal behavior of the material, enabling a comprehensive understanding of how the microstructure evolves under different thermal conditions. This is essential for optimizing processing parameters and tailoring the material properties for specific applications.
The in-situ transformation behavior of high-carbon steel overspray powders was investigated using calorimetry. The decomposition of austenite, austenitization temperature, and martensite formation could be recorded.
In-situ thermogravimetric methods can track gaseous uptake or release during heat treatment, which remains an important topic with interstitial hardening of martensitic steels.
In the case of 420 stainless steel, both grain structure and phase constituents varied as a function of nitrogen gradient through the sample. In particular, the martensite morphology demonstrated variation based on local nitrogen content.
17-4PH produced through LPBF in N2 cover gas and nitrogen gas-atomized powder will maintain a primarily austenitic microstructure, despite having a lower nitrogen content than the nitrogen- loaded wrought 17-4PH, which still displayed a martensite transformation. This may be due to additional microstructural differences in the as-fabricated condition.
Excess nitrogen has a significant impact on the martensite start temperature of stainless steels and promotes retained austenite in the final microstructure. As shown through dilatometry and microstructural analysis, an increased nitrogen content results in a suppressed Ms temperature.
Acknowledgements
We would like to express our gratitude to Asgaard Metals for their invaluable support in providing high- carbon steel for our research.
Bibliography
- P. Bajaj, A. Hariharan, A. Kini, P. Kürnsteiner, D. Raabe, and E. A. Jägle, “Steels in additive manufacturing: A review of their microstructure and properties,” Mater. Sci. Eng. A, vol. 772, p. 138633, 2020, doi: https://doi.org/10.1016/j.msea.2019.138633.
- G. B. Bang et al., “Microstructural and mechanical properties of AISI 440C stainless steel fabricated using selective laser melting,” Mater. Sci. Eng. A, vol. 860, p. 144259, Dec. 2022, doi: 10.1016/j.msea.2022.144259.
- V. M. Rodríguez, V. H. López-Morelos, V. K. Nadimpalli, D. Bue Pedersen, A. Ruiz, and M. A. J. Somers, “Effect of Heat Treatment Processes on the Microstructure and Mechanical Properties of Spray‐Formed 440C Martensitic Stainless Steel,” Steel Res. Int., vol. 94, no. 6, Jun. 2023.
- S. H. Mohamed Salleh, M. Z. Omar, and S. Junaidi, “PHASE TRANSFORMATION AND THERMAL ANALYSIS OF MARTENSITIC STAINLESS STEEL 440C.”
- J. S. Park, J. H. Park, M.-G. Lee, J. H. Sung, K. J. Cha, and D. H. Kim, “Effect of Energy Input on the Characteristic of AISI H13 and D2 Tool Steels Deposited by a Directed Energy Deposition Process,” Metall. Mater. Trans. A, vol. 47, no. 5, pp. 2529–2535, May 2016, doi: 10.1007/s11661- 016-3427-5.
- G. Zhang, H. Yuan, D. Jiao, Z. Li, Y. Zhang, and Z. Liu, “Microstructure evolution and mechanical properties of T15 high speed steel prepared by twin-atomiser spray forming and thermo-mechanical processing,” Mater. Sci. Eng. A, vol. 558, pp. 566–571, Dec. 2012, doi: 10.1016/j.msea.2012.08.050.
- M. A. S. bin A. Rahim, M. bin Minhat, N. I. S. B. Hussein, and M. S. bin Salleh, “A comprehensive review on cold work of AISI D2 tool steel,” Metall. Res. Technol., vol. 115, no. 1, Art. no. 1, 2018, doi: 10.1051/metal/2017048.
- F. Deirmina, N. Peghini, B. AlMangour, D. Grzesiak, and M. Pellizzari, “Heat treatment and properties of a hot work tool steel fabricated by additive manufacturing,” Mater. Sci. Eng. A, vol. 753, pp. 109–121, Apr. 2019, doi: 10.1016/j.msea.2019.03.027.
- F. Großwendt et al., “Additive manufacturing of a carbon-martensitic hot-work tool steel using a powder mixture – Microstructure, post-processing, mechanical properties,” Mater. Sci. Eng. A, vol. 827, p. 142038, Oct. 2021, doi: 10.1016/j.msea.2021.142038.
- W. Shan and Y. Lin, “T15 High Speed Steels Produced by High-Temperature Low-Pressure Short- Time Vacuum Hot-Pressing Combined with Subsequent Diffusion-Bonding Treatment,” Metals, vol. 13, no. 5, Art. no. 5, May 2023, doi: 10.3390/met13050998.
- H. Berns, R.L. Juse, J.W. Bouwman, B. Edenhofer, Solution Nitriding of Stainless Steels – “A New Thermochemical Heat Treatment Process*”, T. Bell and K. Akamatsu, Eds., Stainless Steel 2000: Thermochemical Surface Engineering of Stainless Steel. London: CRC Press, 2020. doi: 10.1201/9780367814151.
- T. L. Christiansen, M. Villa, C. Tibollo, K. V. Dahl, and M. A. J. Somers, “High Temperature Solution Nitriding of Stainless Steels; Current Status and Future Trends∗,” vol. 75, no. 2, pp. 69–82, 2020, doi: 10.3139/105.110406.
- M. Pozuelo, J. E. Wittig, J. A. Jiménez, and G. Frommeyer, “Enhanced Mechanical Properties of a Novel High-Nitrogen Cr-Mn-Ni-Si Austenitic Stainless Steel via TWIP/TRIP Effects,” Metall. Mater. Trans. A, vol. 40, no. 8, pp. 1826–1834, Aug. 2009, doi: 10.1007/s11661-009-9863-8.
- N. Saenarjhan, J.-H. Kang, and S.-J. Kim, “Effects of carbon and nitrogen on austenite stability and tensile deformation behavior of 15Cr-15Mn-4Ni based austenitic stainless steels,” Mater. Sci. Eng. A, vol. 742, pp. 608–616, Jan. 2019, doi: 10.1016/j.msea.2018.11.048.
- H. W. Lee, J. H. Kong, D. J. Lee, H. Y. On, and J. H. Sung, “A study on high temperature gas nitriding and tempering heat treatment in 17Cr–1Ni–0.5C,” Mater. Des., vol. 30, no. 5, pp. 1691– 1696, May 2009, doi: 10.1016/j.matdes.2008.07.023.
- ASTM, “A564,” ASTM International, West Conshohocken, PA, 2021.
- E. A. Lass, M. R. Stoudt, and M. E. Williams, “Additively Manufactured Nitrogen-Atomized 17-4 PH Stainless Steel with Mechanical Properties Comparable to Wrought,” Metall. Mater. Trans. Phys. Metall. Mater. Sci., vol. 50, no. 4, pp. 1619–1624, 2019.
- R. Bhambroo, S. Roychowdhury, V. Kain, and V. S. Raja, “Effect of reverted austenite on mechanical properties of precipitation hardenable 17-4 stainless steel,” Mater. Sci. Eng. A, vol. 568, pp. 127–133, 2013, doi: https://doi.org/10.1016/j.msea.2013.01.011.
- M. Villa and M. A. J. Somers, “Cryogenic treatment of an AISI D2 steel: The role of isothermal martensite formation and ‘martensite conditioning,’” Cryog. Guildf., vol. 110, p. 103131, 2020, doi: 10.1016/j.cryogenics.2020.103131.
- D. A. Porter, K. E. Easterling, and M. Y. Sherif, Phase Transformations in Metals and Alloys, 4th ed. CRC Press, 2022.
- M. Umemoto, E. Yoshitake, and I. Tamura, “The morphology of martensite in Fe-C, Fe-Ni-C and Fe- Cr-C alloys,” J. Mater. Sci., vol. 18, no. 10, pp. 2893–2904, Oct. 1983, doi: 10.1007/BF00700770.
- G. Krauss, “Martensite in steel: strength and structure,” Mater. Sci. Eng. A, vol. 273–275, pp. 40–57, Dec. 1999, doi: 10.1016/S0921-5093(99)00288-9.
- L. D. Barlow and M. Du Toit, “Effect of Austenitizing Heat Treatment on the Microstructure and Hardness of Martensitic Stainless Steel AISI 420,” J. Mater. Eng. Perform., vol. 21, no. 7, pp. 1327– 1336, Jul. 2012, doi: 10.1007/s11665-011-0043-9.
- S. Cheruvathur, E. A. Lass, and C. E. Campbell, “Additive Manufacturing of 17-4 PH Stainless Steel: Post-processing Heat Treatment to Achieve Uniform Reproducible Microstructure,” JOM, vol. 68, no. 3, pp. 930–942, Mar. 2016, doi: 10.1007/s11837-015-1754-4.
Printed with permission of the copyright holder, authors. Statements presented in this paper are those of the authors and may not represent the position or opinion of the American Gear Manufacturers Association. (AGMA) This paper was presented October 2024 at the AGMA Fall Technical Meeting. 24FTM01