There have been many myths concerning the quenchants used by ancient blacksmiths in the heat treatment of swords and knives. Various liquids have been cited in the archeometallurgical literature as quenchants. Each of these quenchants is supposed to extend to the knife’s special and even mythical properties. Some possible quenchants used by ancient blacksmiths will be discussed.
Introduction
Much of the early history of quenching is unknown prior to 1500 AD because it wasn’t written down. There have been some texts written throughout the past that hinted at quenchants used, but very few detailed the process.
Much of the history of quenching is interlaced with the early production of iron. Probably one of the earliest references to smelting and blacksmithing is from the Old Testament in Genesis 4:22 [1] where Tubal-Cain is mentioned as “an artificer of bronze and iron.”
It is not known when steel was first created, or who first created steel. It is suggested from tradition (Herodotus, Xenophon, and Strabo) and archaeological evidence [2] [3] that ironworking developed in the Middle East, in Turkey, near the plateau of Anatolia in 1400–1200 BC by the Hittites [4] [5]. Iron smelting was well known by the second millennium, and described by Homeric poems (880 BC), the History of Herodotus (446 BC), and Aristotle [6] (350BC). Because of ore variation, and the skill of the individual craftsman, the production of steel was often poor quality and limited in production [7].
One of the first mentions of quenching is from Homer (circa 800 BC):
“As when a man who works as a blacksmith plunges a screaming great axe blade or adze into cold water, treating it for temper, since this is the way steel is made strong, even so Cyclops’ eye sizzled about the beam of the olive ….” Odyssey 9.389-9394, translation by R Lattimore
This dramatic image of quenching indicates familiarity with the concept of quenching of steel. But, much of the history of quenching has been shrouded in mystery and magic.
Quenching myths
One of the earliest myths perpetrated regarding quenching ancient steel was the idea that slaves or virgins were used as a quenching medium. The idea being that the hot sword or knife plunged into the body of a slave would impart special properties. Slaves as a quenching medium was first presented with a tongue-in-cheek reference by Douglas Fisher [8]. This was previously noted by John Sullivan [9]. From a practical perspective, it is not likely that slaves or virgins were used as a quenching medium. The physical force required to plunge a blade while hot would likely cause the blade to deflect and warp due to Euler Column Theory [10]. Further, it is likely that the slaves would tend to writhe about, causing distortion to the blade. Lastly, slaves and virgins are not renewable. Any large production would certainly exceed the available supply.
In the first millennium, few technological advances were made in Europe. Some Icelandic sagas spoke of searching through many kingdoms to find the proper water to harden the sword Ekkisax and weapons that are hardened in blood [11]. Predominantly, the advances in metallurgical technology were located in the Arab world, India, China, and Japan. While European armor blacksmiths were improving and gradually perfecting their craft, the Crusaders of the 12th century had no steel that was the equal of Islamic metallurgy. The Japanese sword was even better than the Islamic sword by an even greater margin [12].
In an account of Second Captain Massalski [13], it was indicated the Persians quenched their steel in pre-heated hemp oil. He further indicated that grease and bone marrow were sometimes added to the quenchant:
“If it is a dagger, it is held flat; if it is a sabre, it is quenched little by little, beginning by the end of the cutting edge, holding the latter toward the bath. This manoeuvre is repeated until the oil stops smoking, which proves that the blade has cooled. After quenching, the blade is always soiled with burnt oil. This dirt is removed by heating it enough to set light to a piece of wood and by rubbing with a rag from a bedsheet.” — English Translation by Graham Cross.
Pretextat-Lecomte is a French painter and mosaic artist who lived at the end of the 19th century and was invited to Istanbul for the restoration and reconstruction of some historical art pieces. He spent many years in Istanbul, and from his studies of oriental arts, he wrote the “Arts and Crafts of the Orient” in 1902. (Les Arts et Metieres de la Turquie de l’Orient, published in Paris in 1902) [14]. He described the process of quenching a blade:
“… Now it was required to quench it in order to give it the necessary strength, and that was the interesting point of the procedure: Europeans quench the steel in water, vegetable oil, or cattle fat, but in the East they were doing it on air. When the craftsmen were done with the processing of the metal, they heated it until totally red and gave it to a cavalry man waiting on his horse, ready for a ride. The cavalry man rode his horse in the wilderness, waving the blade in the air with crazy screams to make his horse ride faster.”
On the other hand, “air quenching” was certainly not the only method for making “superior steel” in the Ottoman period. Kemankes (translation is Bowman) Mustafa Aga gives a special formula in his book, The Book of Arrow [15], for making armor-piercing arrowheads and sword blades. His quenching medium consists of:
- 1 okka Quick Lime (CaO)
- 1/2 okka Soda (NaCO)
- 1/2 okka Carbonas Cupricus (Copper Oxide?)
- 1/2 okka Arsenic Sulphate (AsS)
- 2 okka Radish juice
- 1 okka Wild Onion juice
- 1/2 okka Valonia ash
- 1 okka Tar
(Okka is a weight unit and corresponds to 1,283 grams.)
Chinese quenching
The earliest known Chinese word for quench-hardening is cui [16] and is still used in the modern term for quenching cuihuo [16]. Water was predominately the preferred quenchant:
“When a skilled metallurgical worker ‘casts’ [zhu] the material of a Gan Jiang [sword], quench-hardening [cui] its tip with pure water and grinding its edge with a whetstone from Yue, then in the water it can slice water-dragons, and on land it can cut rhinoceros hide as quickly as sweeping and sprinkling or drawing in mud.” — Sheng zhu dexian chen song presented to the Emperor Xuan-di (73 BC to 49 BC) by Wang Bao [16]
There is some thought that the idea of quenching was a Han Dynasty innovation [16]. Early Tang texts indicated the Yunnan quench-hardened steel in “the blood of a white horse” [16]. Various texts indicate that different waters were good for quenching, while others were inadequate. The Qingzhand and the Longguan Rivers were noted for being good for quenching [16]:
“The Han River is sluggish and weak and is not suitable for quench-hardening. The Shu River is bold and vigorous …”
Quenching in vinegar was poor practice “making it brittle and easy to break” [16]. It seems that quenching in urine was a common practice, with quenching in the urine of five sacrificial animals or the fat of five sacrificial animals. It was given that “such a sword could penetrate 30 layers of armor” [16].
There was also an understanding of the effects of different quenchants and the effect on performance. In 6 AD, the blacksmith Qiwu Huaiwen used animal urine and animal grease to affect different quench rates. The characters used differentiated like this: Cui was denoting quenching in animal grease, while yu was designated for quenching in urine. Song Yingxing discusses quenching in oil, which provides a softer quench, “since the strength of steel lies in quenching.” Further, it was noted that barbarians quench in di son, the “urine of the earth,” a kind of oil not produced in China [16]. This perhaps is the first possible mention of quenching in petroleum-based oils.
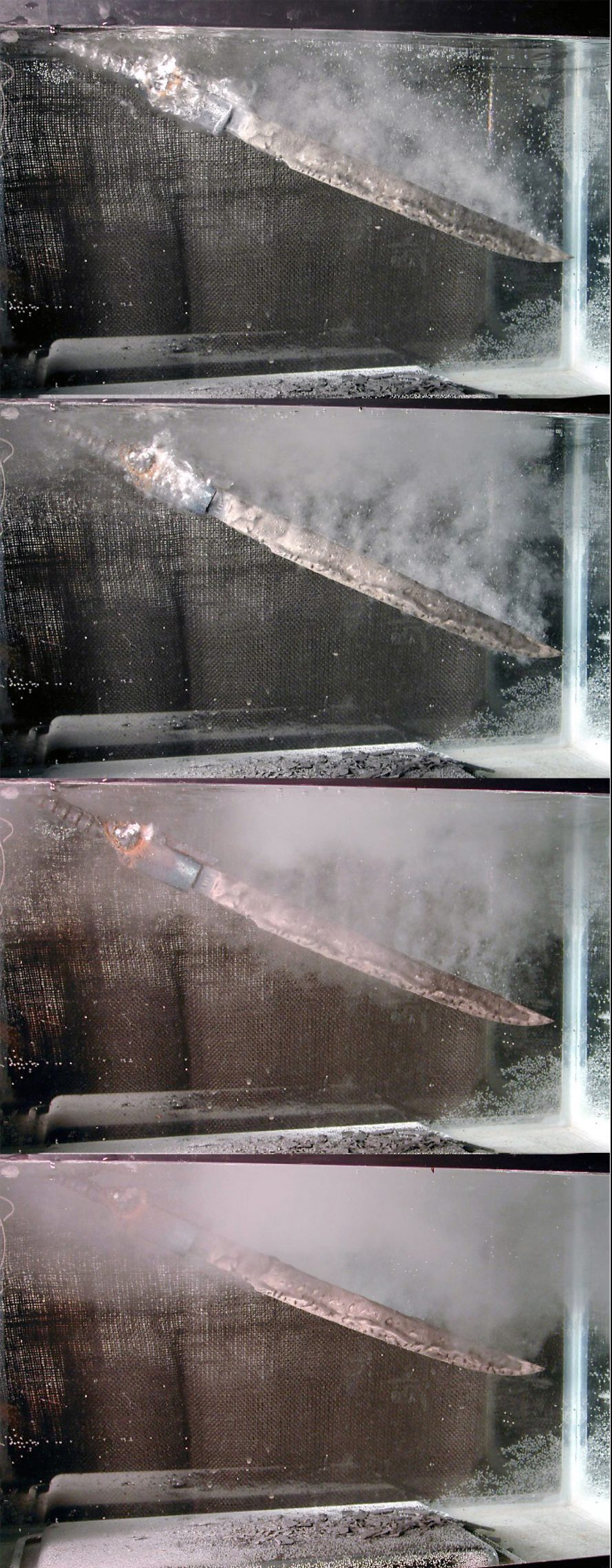
Advanced methods in Japan
The metallurgical state-of-the-art was very advanced in Japan. The science and craftsmanship of the Japanese sword is still revered today for being beautiful and effective, capable of maintaining a sharp edge and the unique curve of the blade.
Swords made by the traditional method are manufactured from steel produced by the tatara method. This steel, or tamahagane, is produced from iron sands that have very low phosphorus and sulfur.
The basic process is like that practiced by the Europeans in the fifth to sixth century AD. The sharp edge consists of high-carbon steel to retain an edge, and the interior of the blade consists of lower carbon steel for toughness and ductility. However, the Europeans immersed the entire sword in the water, with the entire surface of the sword quenched rapidly. In the Japanese method, controlled quenching is achieved at specific rates at different locations on the blade.
Prior to heating, the Japanese sword maker applied a closely guarded secret clay mixture (called yakiba-tsuchi), that consisted of stone powder, clay, and charcoal. The stone powder helps prevent the clay from cracking during heating of the blade; the charcoal is burned out during heating, producing a site for initiation of nucleate boiling, depressing the formation of the vapor phase. The thickness of the clay determines the quench rate. The clay is thinnest at the edge of the blade and thickest at the ridge of the blade — opposite the edge. The blade is immersed in water in the water box or mizubune. The edge is quenched with the highest heat-transfer rate and produces martensite, while the ridge experiences a much milder quench and transforms to a mixture of pearlite and ferrite. The interface between the pearlite and martensite is called the hamon.
This unique and ingenuous method of quenching also produces the characteristic curvature of the blade. As the blade is quenched, the edge contracts, and reverse bending occurs, called gyaku-sori. At the martensite transformation, the sori, or normal bending occurs, due to the volumetric transformation of martensite. Gyaku-sori appears again at the pearlite transformation at the ridge of the blade. Finally, the final curvature or sori appears as the pearlite contracts due to thermal contraction, contributing to strong compressive residual stress at the blade edge. Final tempering of the blade, or aidori, is done in a charcoal fire. This understanding of the quenching process, practiced since the fifth to sixth century, shows the advanced nature of the Japanese metalsmiths.
 Medieval quenchants
Medieval quenchants
Focusing on Europe, and specifically early medieval Anglo-Saxon and Viking knife manufacture, several quenchants were identified. Some Icelandic sagas spoke of searching through many kingdoms to find the proper water to harden the sword Ekkisax and weapons that are hardened in blood [17]. Probably the first significant work in medieval Europe was written by Theophilus [18] (1125), a 12th century German Monk. The “Diver Arts” describe several good quenchants. His recommendations for quenchants were very specific:
“Tools are also given a harder tempering in the urine of a small, red-headed boy than in ordinary water.” [18]
Other recommendations for quenchants included the urine of goats fed ferns for three days.
Giambattista della Porta (ca. 1535-1615) in his books Natural Magic [19] showed an excellent understanding of the reason why many quenchants were effective, and some of the underlying principles:
“If you quench red hot iron in distilled vinegar, it will grow hard. The same will happen, if you do it into distilled urine, by reason of the salt it contains in it. If you temper it with dew, that in the month of May is found on vetches leaves, it will grow most hard. For what is collected above them, is salt, as I taught elsewhere out of Theophrastus. Vinegar, in which Salt Ammoniac is dissolved, will make a most strong temper. But if you temper Iron with Salt of Urine and saltpeter dissolved in water, it will be very hard. Or if you powder Saltpeter and Salt Ammoniac, and shut them up in a glass vessel with a long neck, in dung, or moist places, till they resolve into water, and quench the red hot Iron in the water, you shall do better. Also iron dipped into a Liquor of Quicklime, and Salt of Soda purified with a Sponge, will become extreme hard. All these are excellent things, and will do the work.”
There was also an understanding of the cause of quench cracking, and the results of quenching in other than water for Porta’s “The Temper for Instruments to let blood”:
“It is quenched in oil, and grows hard, because it is tender and subtle. For should it be quenched in water, it would be wrested and broken.”
Various authors describe other quenchants including: pigeon droppings, flour, honey, olive oil, and milk [20] [21] [22]. Other quenchants, including urine, water and solubilized animal fats and whale oil, are described by Smith [23], Biringuccio [24], Agricola [25], and others:
“Take clarified honey, fresh urine of a he-goat, alum, borax, olive oil, and salt; mix everything well together and quench therein.”
Von Stahel and Eysen (1532) [26] also discussed quenchants to be used for heat-treated parts:
“Take varnish, dragon’s blood, horn scrapings, half as much salt, juice made from earthworms, radish juice, tallow, and vervain and quench therein. It is also very advantageous in hardening if a piece that is to be hardened is first thoroughly cleaned and well-polished.”
Other modern researchers [27] [28] have indicated that oil, milk, urine, and blood have been used as quenchants.

Cooling curves of various quenching media
A paper by MacKenzie and Graham [29] evaluated the cooling curves of synthetic urine, water, beer, milk, and bovine blood.
The synthetic urine was a very fast quenchant and greatly exceeded the cooling rate of water. This is caused by the salts precipitating on the surface of the probe, resulting in nucleation sites for nucleate boiling to occur. This cooling curve was like commercial quenchants containing inorganic salts.
The water quench is like the synthetic urine, but much slower. Some stable vapor phase is present.
Beer showed a quench rate like a fast-accelerated quench oil, with a similar maximum cooling rate. However, a long-extended vapor phase occurred due to the residual carbonation present in the beer. This acts to stabilize the vapor phase.
Milk showed a moderate vapor phase, with a slow overall maximum cooling rate. This is thought to be the result of the butter fat present in the quenchant.
Blood showed a very unusual cooling curve. There was an initial vapor phase like the other quenchants; however, this was followed by a very long and prolonged stable cooling rate from approximately 750°C to 100°C. Virtually no nucleate boiling occurred. In a typical cooling curve, virtually all the quenchants reached nearly ambient temperature within 60 seconds. Blood on the other hand, at the end of the 60 seconds, the probe was at a temperature of 341°C. It took three minutes for the cooling curve probe to reach 80°C. This is an extremely slow cooling curve. It was thought that quenching cooked the fat and blood insulated the probe, slowing heat transfer. This insulating later drastically slowed the heat-extraction rate.
Conclusions
There are a lot of hyperbole and myths regarding ancient heat treatment and quenching. Much is simply wrong. While blood was claimed to produce magical properties to a blade, it has been shown to have a very slow cooling curve and not capable of hardening the simple steels available to early blacksmiths. Water and urine are really the only quenchants that would be able to heat treat the simple steels [30] heat treated in early times.
It is hoped that this work on possible quenchants used by ancient blacksmiths can further the understanding of the technological and cultural contribution of the first metallurgists.
References
- A. Guillaume, “Metallurgy in the Old Testament,” Palestine Exploration Quarterly, vol. 94, no. July/Dec., pp. 129-132, 1962.
- T. A. Wertime, “Man’s First Encounters with Metallurgy,” Science, vol. 146, pp. 1257-1267, 1964.
- T. A. Wertime, “The Beginnings of Metallurgy,” Science, vol. 182, pp. 857-887, 1973.
- L. Aitchison, A History of Metals, Volume I, London: MacDonald & Evan Ltd., 1960.
- K. A. Yener, “Swords, Armor and Figurines,” Biblical Archaeologist, vol. 58, p. 2, 1995.
- J. N. Friend, Iron in Antiquity, London: Charles Griffin & Co., 1926.
- A. R. Williams and K. R. Maxwell-Hyslop, “Ancient Steel from Egypt,” J. Archeological Sciences, p. 97, 1976.
- D. Fisher, The Epic of Steel, New York: Harper & Row, 1963.
- J. Sullivan, The Story of Metals, Ames, IA: American Society for Metals and The Iowa State College Press, 1951.
- T. H. Megson, Aircraft Structures for Engineering Students,, 7th ed., London: Elsevier B.V., 2022.
- A. G. Drachmann, “De navngivne sværd i saga, sagn og folkevise. Gad, København, 1967 (Studier fra sprog- og oldtidsforskning udgivet af det Filologisk–Historiske”.
- National Academy Supplementary Report of the Committee on the Survey of Materials Science and Engineering, Materials and Man’s Needs: Materials Science and Engineering — Volume I, the History, Scope, and Nature of Materials Science and Engineering, National Academy of Science, 1975.
- Massalski, “Preparation de L’Acier Damasse en Perse,” Annuaire du Journal des mines de Russie, pp. 297-308, 1841.
- Prétextat-Lecomte, Les Arts et Métiers de la Turquie et de l’Orient, Paris: Société d’Éditions Scientifiques, 1902.
- G. K. Alpay, “Lamii Chelebi and His Works,” J. Near Eastern Studies, vol. 35, no. 2, pp. 73-93, 1976.
- D. B. Wagner, “Ferrous Metallurgy,” in Science and Technology in China, vol. 5, Cambridge, Cambridge University Press, 2008.
- A. G. Drachmann, “De navngivne svaerd i saga, saga og folkevise,” in Filogisk-Historiske Samfund, Copenhagen, 1967.
- Theophilus, De Diversis Artibus, C. R. Dodwell, Ed., London: Thomas Nelson & Sons, 1941.
- J. P. Porta, Magiae Naturalis, 1584.
- J. Anstee, “A study in Pattern Welding,” Medieval Archaeology, vol. 5, pp. 71-93, 1961.
- C. S. Smith, Sources for the History of the Science of Steel 1532-1786, Boston, MA: MIT Press, 1986.
- H.-U. Haedeke, Metalwork, Universe Books, 1970, p. 227.
- C. S. Smith, A History of Metallography: The development of Ideas of the Structure of Metals before 1890, Cambridge, MA: MIT Press, 1988.
- V. Biringuccio, Pirotechnia, Toronto, Canada: General Publishing Company, 1990.
- G. Agricola, De Re Metallica, New York, New York: Courier Cover Publications, 1950.
- Von Stahel und Eysen, 2002.
- R. F. Tylecote, Scientific Examination and Analysis of Iron Objects, Oxford: Oxford University Press, 1990, pp. 140-154.
- R. Maddin, “The Early Blacksmith,” in Symposium of the UISPP Comite’ pour la Sidrurgie Ancienne, Belfast, 1987.
- D. S. MacKenzie and G. Graham, “Beer, Blood, and Urine – Mythological Quenchants of Ancient Blacksmiths.,” in IFHTSE 2016: Proceedings of the 23rd IFHTSE Congress (April 18-21), Savannah, GA, 2016.
- E. Blakelock, PhD Dissertation: The Early Medieval Cutting Edge of Technology: An Archaeomettallurgical, technological and social study of the manufacture and use of Anglo-Saxon and Viking iron knives, and their contribution to the early medieval economy, Bradford, West Yorkshire: University of Bradford, 2012.