At the turn of the 20th century, the use of molten salt as a heating and quenching medium for steels was developed in England. It rapidly came into use in Europe as a low-cost method of heat treating. Equipment was inexpensive, and molten salt provided a reproducible method of heat treatment.
In this short article I will review the types of salts used for heat treating and some hazards associated with molten salt heat treatment.
Advantages of Molten Salt Heat Treating
Parts that are heated in salt baths are heated by conduction. The thermal mass of the molten salt provides a large thermal source for heating of parts. Heating of parts in a salt bath is much more rapid than in air or atmosphere furnace. The heat-up of the parts is only limited by the thermal conductivity of the part. Since metals have high thermal conductivity, the core of the part lags the surface by only a small amount. A 25 mm diameter of steel would require four minutes to reach the austenitizing temperature, while it would take 30 minutes or more in a standard radiation furnace [1].
Salt bath furnaces are efficient at heating parts, with more than 90 percent of the heat applied to the furnace going directly to the part. Atmosphere furnaces are approximately 60 percent efficient. Temperature uniformity is excellent, with uniformity less than ± 3°C throughout the bath.
When parts are heat treated in a molten salt, they are completely immersed. Surface protection from the salt can reduce decarburization and the formation of scale.
Quenching in a molten salt can reduce distortion. Since heat transfer is by conduction, a much more uniform heat transfer occurs across the surface of the part. Further, due to buoyancy effects, sagging of parts during heat treating is minimized.

Types of Salts – Salt Composition
There are several different types of heat-treating operations that can be performed in molten salts. The first is to solution heat treat the part (austenitize in steel) to bring it to the desired temperature. The second operation is to quench the part in the molten salt as part of martempering operation or austempering. Lastly, there is the tempering or aging operation (non-ferrous alloys). All these unit operations can be performed in a molten salt.
There are also specialized salts used for carburizing or nitriding operations. These usually contain a form of sodium cyanide or cyanate. These salts will be mentioned briefly, as their use has significantly declined due to environmental and safety considerations.
Molten salts can be used at a temperature range of 150°C to 1,287°C (300°F to 2,350°F). Molten salts are divided into three temperature ranges, with characteristic chemistry. The ranges are low temperature (150° – 620°C); medium temperature (650° – 982°C); and high temperature (982°C – 1,287°C) molten salts.
Low Temperature (150°–620°C)
Low temperature salts are used for a wide variety of unit operations, such as solution heat treating of aluminum, or martempering and austempering of steels. They are usually mixtures of various nitrates and nitrates of sodium and potassium. These salts are completely molten at 150°C. Some salts, used for energy storage in solar applications, are molten at 121°C (250°F. These salts contain up to 25 percent lithium nitrate. There are no known heat-treating applications using lithium nitrate as a component of the low temperature salt due to the high cost of lithium salts.
Use of these mixtures of nitrate/nitrite baths at temperatures above 620°C is not recommended. These baths are unstable above 620°C, and a strong exothermic reaction can result. This is particularly true when in contact with aluminum.
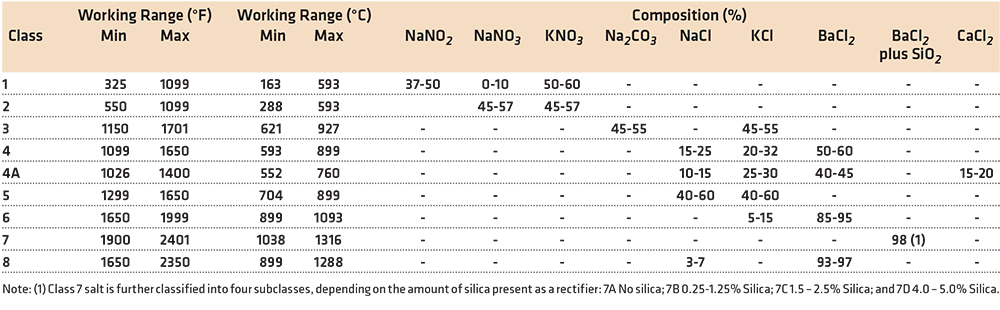
Medium Temperature (650°–982°C)
Since nitrates and nitrites are unstable at temperatures above 620°C, another type of molten salt chemistry is required. Molten salts in this class of salts are mixtures of sodium chloride (NaCl), potassium chloride (KCl), calcium chloride (CaCl2), sodium carbonate (Na2CO3), and barium chloride (BaCl2). At lower temperatures within the operating range, the composition would be primarily potassium chloride and sodium carbonate. At the top end of the range, barium chloride would be the primary constituent. For most general-purpose heat treating to 900°C, a 50/50 mix of sodium chloride and potassium chloride is the most common. This avoids any environmental or disposal issues with barium salts.
While these salts are neutral to steel, they will decarburize steels as the salts oxidize. As temperature is increased, the salts will tend to oxidize more rapidly. Initially, the chlorides form oxides in the form of Na2O or K2O. This occurs due to the presence of air at the liquid interface. Formation of sodium carbonate eventually occurs, with further increases in decarburization. The use of a rectifier, such as borax, boric acid, silicon carbide, or ammonium chloride [2], added to the bath can restore the neutral character of the bath. Previously, methyl chloride was used as a rectifier, by bubbling the methyl chloride through the bath [3]. However, due to health and safety concerns, the use of methyl chloride has largely been discouraged.
High Temperature (982°C–1287°C)
The high temperature molten salt baths are predominately barium chloride, with either potassium chloride or sodium chloride added to the composition. However, the most common bath is barium chloride with a small amount (up to 5 percent) of silica added as a bath rectifier.
Specialized Salts – Nitriding and Carburizing
There are also specialized salts for molten salt carburizing and nitriding, as well as salts for blackening or bluing steels.
In carburizing molten salts, the salts consist of a mixture of barium chloride and sodium cyanide. Concentration of sodium cyanide is on the order of 30-35 percent [4]. Temperatures used are like those used in atmosphere carburizing (850° to 950°C). The depth of carburizing is generally low at 0.5 mm. Because of the relatively shallow carburizing depths, the corresponding time at temperature is short. The concentration of the sodium cyanide can be increased if the depth of carburized case is deeper. Since the cyanide is the source of carburizing, it will deplete over time. To prevent depletion, a cover of carbon is usually added. This prevents oxidation of the cyanide and prevents radiation heat losses. Typical drag-out of about 6 percent means that fresh salt must be added daily.
Obviously, the presence of sodium cyanide can be a serious health and environmental problem if the necessary steps are not taken to mitigate possible release of hydrogen cyanide. Cyanides will decompose in a bath to carbonates [5]. Some hydrogen cyanide may be produced during this decomposition. Therefore, it is necessary that strong mechanical exhaust be used [5]. The storage of any sort of acids should be prohibited from areas where the carburizing salt is stored. If there are acids used in the production process, such as pickling, it is critical that the parts are thoroughly washed and rinsed prior to the acid-containing process. Mechanical ventilation is strongly recommended.
The cyanide wastes (used salt or contaminated rinse water) must be chemically decomposed before they are disposed. Decomposition is usually done by treating the salt in aqueous solution with an oxidizing agent such as sodium hypochlorite [5]. Strong ventilation is required.
Conclusions
In this column, the advantages of the use of molten salt were illustrated. The salt composition of the various temperature ranges of salt were explained. In later columns, the application of molten salt quenching (martempering and austempering) will be discussed.
As always, should you have any comments or questions regarding this column, please contact the editor or myself.
References
- W. J. Laird, “Salt Bath Equipment,” in ASM Handbook 4: Heat Treating, Metals Park, OH, ASM International, 1991, pp. 475 – 483.
- R. W. Foreman, “Pelletized Rectifier for Molten Neutral Salt Baths and Method”. USA Patent 4,009,112, 22 February 1977.
- P. H. Kramer, “Method of Rectifying a Neutral Molten Salt Treating Bath”. USA Patent 2,474,680, 28 June 1949.
- H. Solakain, “Salt baths and Salt Bath Furnaces,” Metal Treating, no. January-February, pp. 3-8, 1958.
- Park Thermal International, Inc., “A Guide to the Safe Use of Molten Salt Baths,” Park Thermal International, Inc., Georgetown, ON Canada, 1996.
- “MIL-S-10699B, Military Specification: Salt, Heat Treating (for Metals),” 1977.