
In the last column, I discussed the effect of temperature on the oxidation of a quench oil. As was alluded to in my last column, the oxidation of quench oil is very dependent on the catalyst used.
Introduction
Quench oils degrade from four primary reasons: oxidation; thermal degradation; contamination; and additive depletion. This is aggravated by residues on parts, washer residues from oil reclaimed from washers, high energy density heater or radiant tubes, and excessive peak temperatures. The addition of robust additive packages prolongs a quenchant life and provides for repeatable quenching.
Oxidation of quench oil is caused by exposure to oxygen. As operating temperatures increase, the kinetics of oxidation approximately double with each 10°C. This is especially true with martempering oils because of their elevated temperature of use.
Thermal degradation is from exposure to temperatures that cause the base oil and additive package to change. This results in the formation of insoluble products of reaction that can cause deposits on parts and sludge the quench tank.
Contamination can be from many sources. Water, dust, scale, and soot are not the direct result of oil degradation but can contribute to other degradation issues. Soot can act as nucleation sites for thermal degradation products and can mimic oxidized oil.
Additive depletion is normal and expected. The antioxidants are consumed as part of their function. Antioxidants are replenished as make-up oil is added.
The oxidation mechanism of quenching oils is very complex [1] [2] [3] [4] [5] [6]. The presence of iron and copper catalyzes the reactions and plays an important part in chain initiation reaction. This is schematically shown in Figure 1.
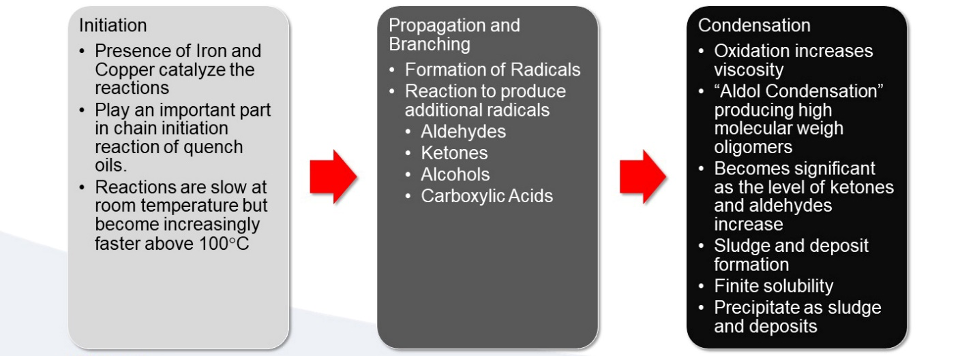
Effect of Catalyst
Often, parts, such as gears, are plated with copper to act as a carburizing “stop-off” to prevent the diffusion of carbon into the part in areas where carburizing is not desired. Copper plating can also be used to protect high-value parts from decarburization of carburizing during neutral hardening. This method is often used for components used in aircraft landing gear. Copper is an effective barrier to carbon diffusion. Copper is also used as a catalyst to accelerate oxidation in testing, such as by ASTM D943 “Standard Test Method for Oxidation Characteristics of Inhibited Mineral Oils.” [7].
In ASTM D 943, a small test cell, that is controlled for temperature, is heated in the presence of a copper coil. An example of this equipment is shown in Figure 2.

Testing [8] was conducted according to ASTM D943 “Standard Test Method for Oxidation Characteristics of Inhibited Mineral Oils.” [7], modified by eliminating the use of water. Standard copper coils were used. To test the effect of different catalysts, the copper coil was plated with nickel. Testing was conducted at 121°C. Oxidation was measured by viscosity (ASTM D445) [8], Total Acid Number (ASTM D664) [9], and by FTIR. Measurements for viscosity and TAN were taken at various intervals. The oil used was a premium martempering oil. The results of the oxidation testing as measured by TAN are shown in Figure 3 and the results of the viscosity testing are shown in Figure 4.
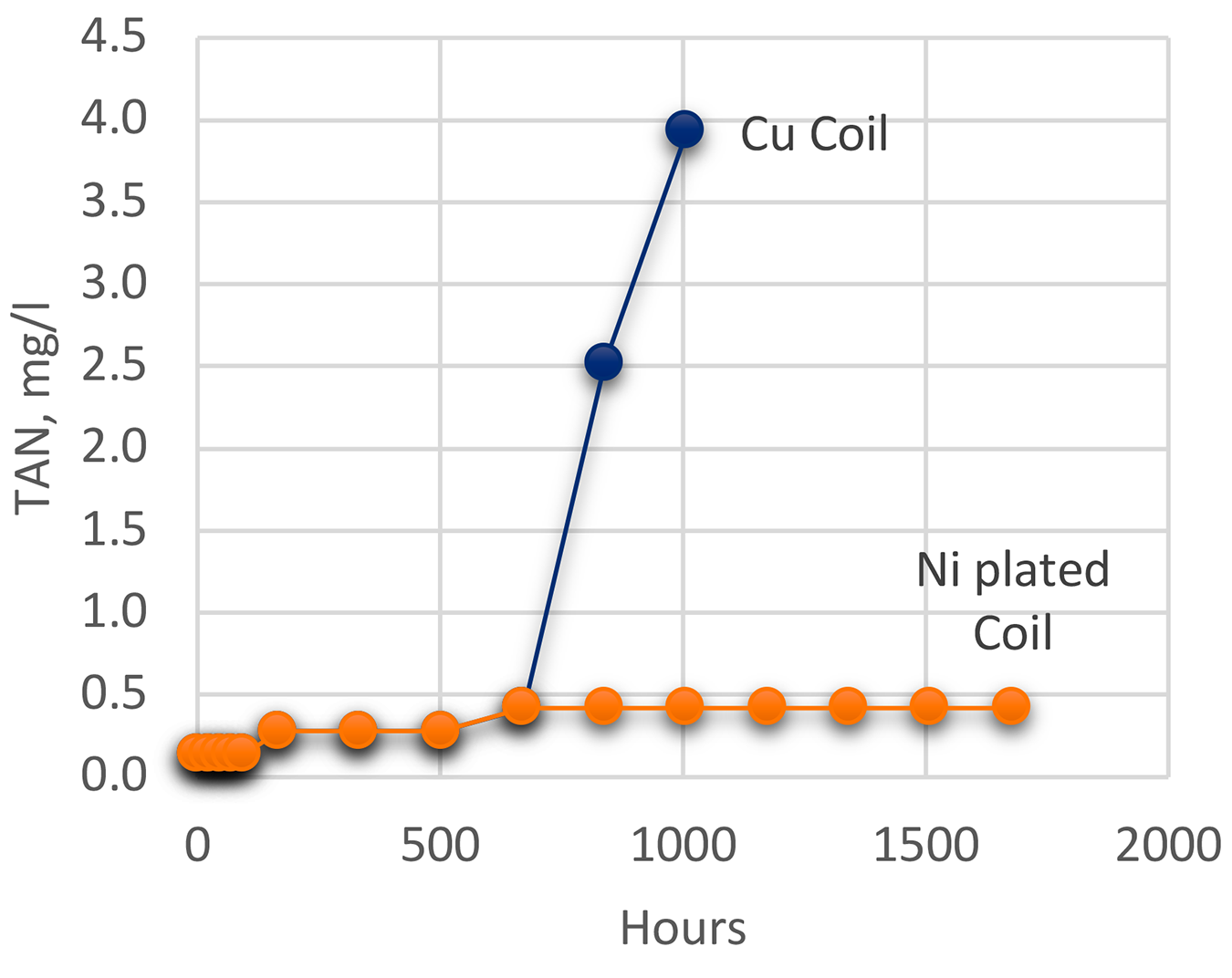
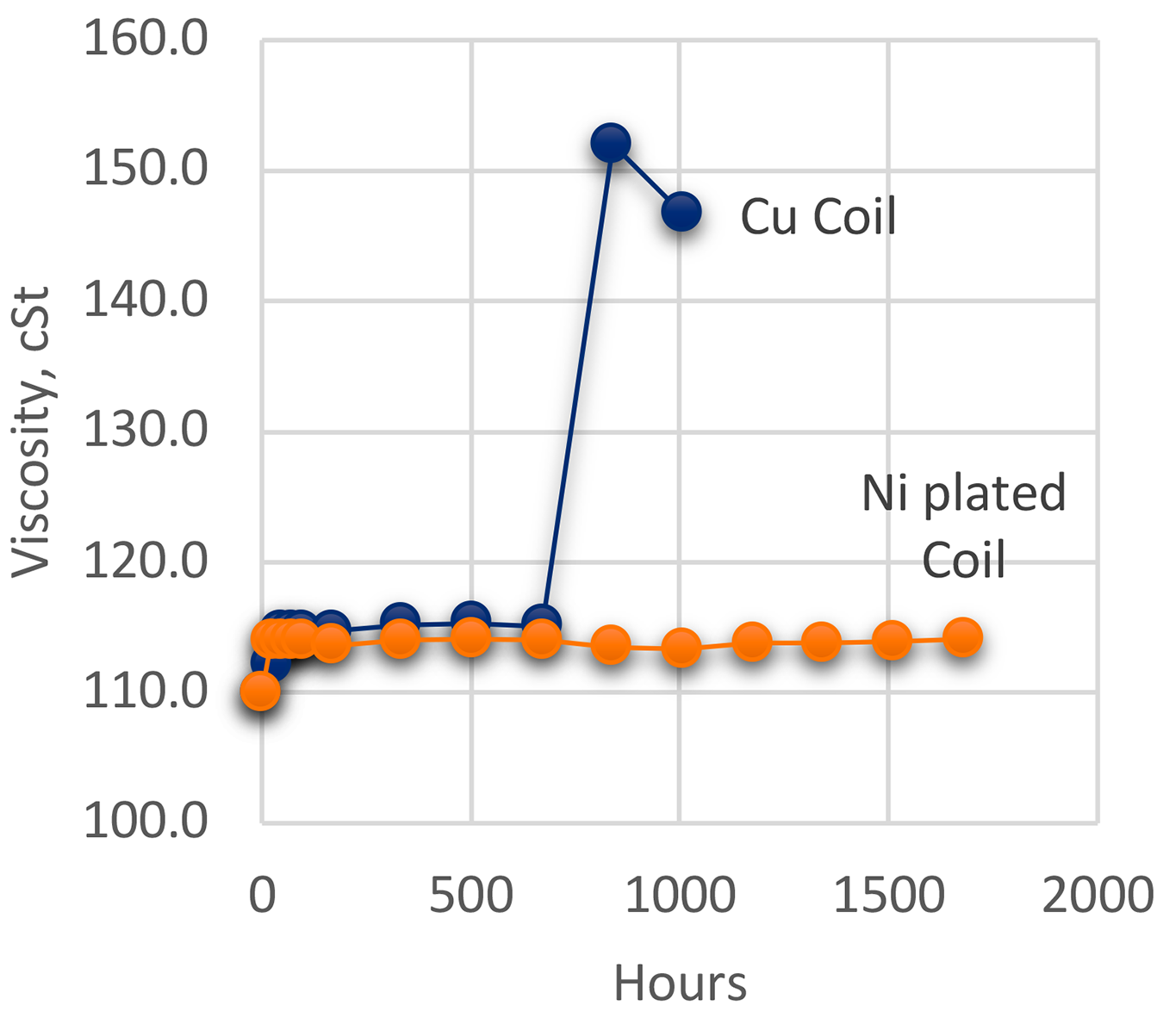
The oxidation testing clearly showed that copper was a very strong catalyst for oxidation of the martempering oil. Nickel plating showed a very mild effect and exhibited very little oxidation during the life of the test. When examined using FTIR (Figure 5), the oil exposed to the copper coil showed extensive oxidation while the oil exposed to the nickel-plated coil showed very little oxidation. The FTIR scans of the new oil and the oil exposed to the nickel coil were practically identical.
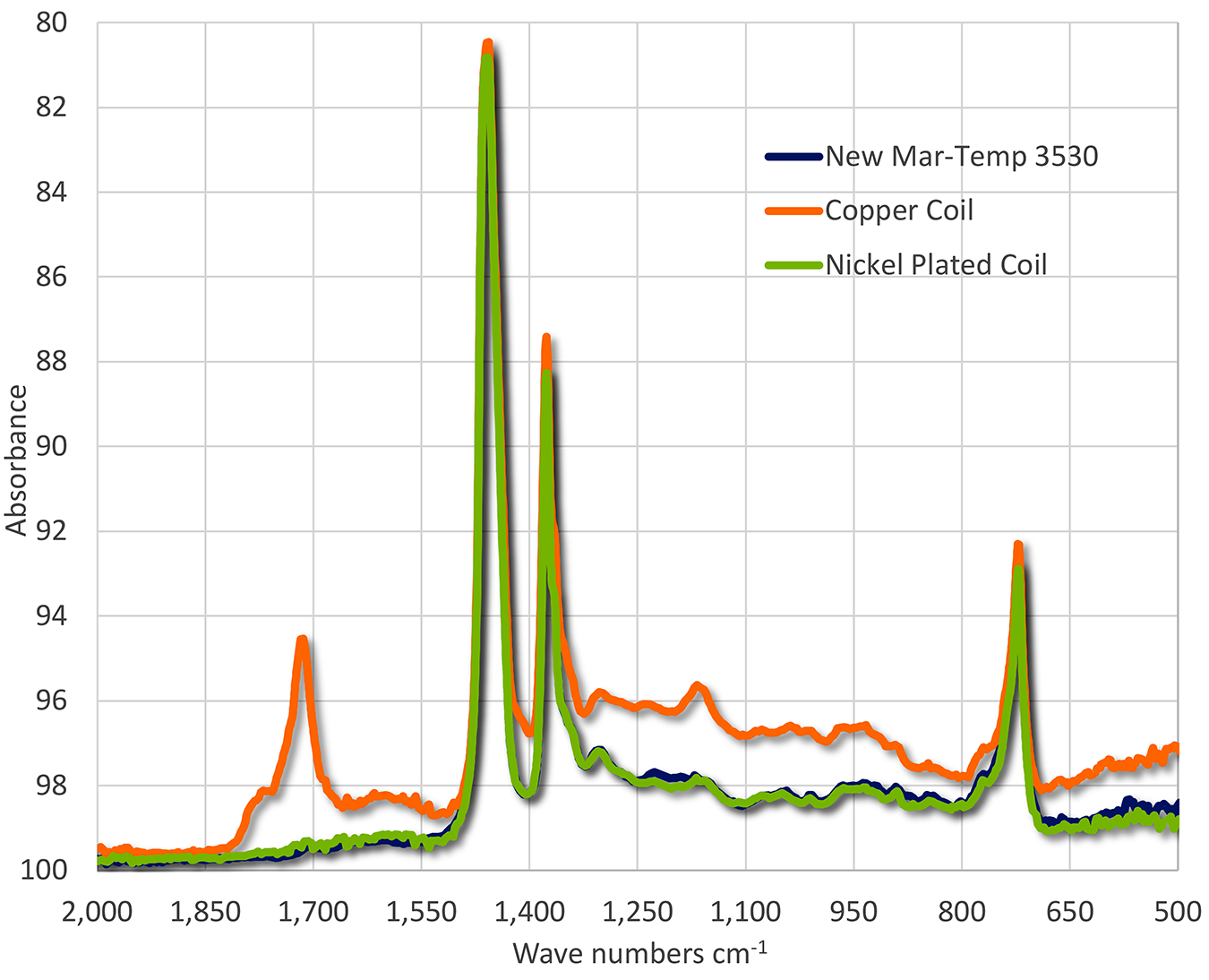
Conclusions
The oxidation testing clearly showed that copper was a very strong catalyst for oxidation of martempering oil. The use of copper as a local prevention of carburization severely increases the oxidation rate of the oil. Nickel plating showed a very mild effect and exhibited very little oxidation during the life of the test. When examined using FTIR, the oil exposed to the copper coil showed extensive oxidation while the oil exposed to the nickel-plated coil showed very little oxidation. The FTIR scans of the new oil and the oil exposed to the nickel coil were practically identical.
The use of nickel plating for the prevention of carburization has merit in the
reduction of oxidation. However, additional testing must be performed to demonstrate that the diffusion of nickel will not change the local composition of the part and change the local hardenability. The use of a copper strike under the nickel plating to improve plating adhesion would help reduce nickel diffusion and provide additional protection from the diffusion of carbon during carburization. Additional investigation is underway to evaluate the effect of nickel plating during high-temperature carburization.
Should you have any comments on this article, or suggestions for further articles, please contact the author or the editor.
References
- T. Colclough, “Lubricating Oil Oxidation and Stabilization,” in Atmospheric Oxidation and Antioxidants, vol. II, G. Scott, Ed., Amsterdam, Elsevier Science B. V., 1993, pp. 1-70.
- V. Gatto, W. Moehle, T. Cobb, and E. Schneller, “Oxidation Fundamentals and Its Application
to Turbine Oil Testing,” J. ASTM, vol. 3, p. 1,
April 2006. - J. March, “The Aldol Condensation,” in Advanced Organic Chemistry, 3rd ed., New York, John Wiley & Sons, 1985, pp. 829-834.
- M. Rasberger, “Oxidative Degradation and Stabilization of Mineral Oil Based Lubricants,” in Chemistry and Technology of Lubricants, R. M. Motier and S. T. Orszulik, Eds., London, Blackie Academic and Professional Publishing, 1997, pp. 98-143.
- G. Scott, Atmospheric Oxidation and Antioxidants, Amsterdam: Elsevier Publishing Company, 1965.
- Y. A. Shlyapnikov, “Antioxidant Stabilization of Polymers,” Russ. Chem. Rev., vol. 50, pp. 581-600, 1981.
- ASTM, “Standard Test Method for Oxidation Characteristics of Inhibited Mineral Oils,” ASTM, 2018.
- D. S. MacKenzie, P. L. Pioli, and J. Kim, “The Effect of Temperature and Catalyst on the Oxidation of Oil used for the Mar-Tempering of Gears,” in IFHTSE 26th Congress, International Congress on Metal Science and Heat Treatment, September 17-19, 2019, Moscow, Russia, 2019.
- ASTM, “ASTM D445: Standard Test Method for Kinematic Viscosity of Transparent and Opaque Liquids (and Calculation of Dynamic Viscosity),” ASTM, 2018.
- ASTM, “ASTM D664: Standard Test Method for Acid Number of Petroleum Products by Potentiometric Titration,” American Society of Testing and Materials International, West Conshohocken, PA.

























