In the early history of the heat treatment of steel, water and brine were used to quench lean alloys that consisted of little more than iron, carbon, and a little manganese. As the use of additional alloying elements such as chromium, nickel, molybdenum, and vanadium increased, water and brine quenchants were simply too fast. Cracking and excessive distortion dictated that slower quenchants be used. Quench oils, with maximum cooling rates from less than 50°C/s to 100°C/s, ruled as the primary steel quenchants.
As environmental regulations, sustainability requirements, and oil prices increased, an alternative to oil was needed (Figure 1). With increased research, the creation of polymer quenchants has not only filled the gap between water and oil quenchants but has replaced oil in many instances.

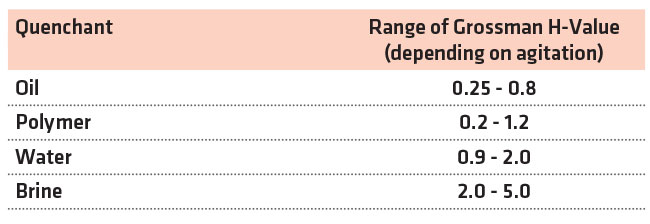
New polymers have been created that achieve the slow quench rate of oil quenchants. Polymer quenchants are being used to quench high hardenability steel forgings, patenting high carbon wire, high carbon railroad rail, and steel castings. Polymer quenchants have been used in mesh belt and integral quench furnaces, as well as typical open quench tanks. Application of polymer quenchants is now extensively used in the heat treatment of aluminum sheet, plate, forgings, and castings to reduce distortion and residual stresses. With proper control of operating variables, polymer quenchants can achieve quench rates that are nearly as fast as brine quenchants, or as slow as straight quench oil that contains no speed improvers. This is illustrated in Table 1.
There are three primary driving forces for the application of polymer quenchants in the heat-treating shop: reduction of fire hazards, environmental concerns, and cost. Water-based quenchants significantly reduce the risk of fire. Environmental concerns, such as disposal and regulations concerning volatile organic compounds (VOC) are reduced due to the use of water and the low volatility of polymer quenchants. The ability of polymer quenchants to be concentrated by reducing water content significantly reduces the disposal costs.
While the cost of polymers per gallon is high compared to oil, polymer quenchants are diluted with water, which drastically reduces their in-tank costs. As an example, a manufacturer had planned to fill his 50,000-gallon open quench tank with moderate speed oil. Filling this tank would cost more than $350,000. An appropriate choice of polymer quenchant at a concentration (15%) that would yield an equivalent quench rate achieved an in-tank cost savings of $175,000. The benefits of reduced fire risk (and lower attendant fire insurance premiums), environmental concerns, and cost have resulted in a much larger market share for polymer quenchants in the past 40 years.
For all polymer quenchants, the primary manufacturing variables to achieve a desired quenching rate are:
- Concentration.
- Agitation.
- Temperature.
As the concentration of the polymer is increased, the effective quench rate is reduced. As the concentration is increased, a limit will be reached where additional additions of polymer will not significantly reduce the cooling rate. This concentration is dependent on the molecular weight of the polymer, and the type of polymer chosen.

Polymer quenchants tend to be more sensitive to agitation than mineral oils. Increasing the agitation increases the cooling rate and reduces the polymer film thickness. However, decreasing the agitation can produce non-uniform quenching because of non-uniform film thickness. It also limits the transport of polymer to the part surface. As in every quenching operation, the magnitude and uniformity of agitation is extremely important. Racking of parts is also more critical in polymer quenchants than mineral oil because of the strong effects of temperature. Agitation tends to minimize these thermal gradients within the quenchant.
Because of the sensitivity of polymer quenchants to agitation, cooling curves of these quenchants are performed using agitation. They are not tested without agitation like oil quenchants or water. Specialized agitation devices are used, such as the Tensi agitation system (Figure 2) as described by ASTM D6482 [1].
The effective quench rate of polymer solutions is affected by temperature. As temperature increases, the quench rate is reduced. Increasing temperature also increases the oxidation and reduces thermal stability of the polymer, effectively shortening the life of the polymer. The amount of degradation is dependent on the amount of polymer present used, and the application temperature. Depending on the polymer used, there is also a limit on the bulk quench temperature of the quenchant, as some quenchants will tend to separate or precipitate from solution. In general, the typical operating temperature range of polymer quenchants is 20-45°C.
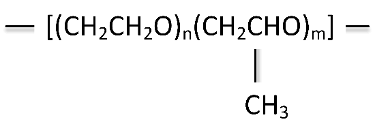
There are four predominant types of polymer quenchants in use today: polyalkylene glycol (PAG), polyethyl oxazoline (PEOX), polyvinyl pyrrolidone (PVP), and sodium polyacrylate (ACR).
1 Polyalkylene glycol quenchants
Polyalkylene glycol (PAG) quenchants are the most-used polymer quenchants in the heat-treating market today. Polyalkylene glycols (PAG), or polyalkylene glycol ethers, were first introduced as quenchants in the early 1970s. PAG quenchants are an example of a copolymer. This quenchant is derived from two monomeric units, ethylene oxide and propylene oxide, to form polyalkylene glycol (Figure 3).
By varying the molecular weights and the ratio of oxides, polymers having broad applicability may be produced. Proper selection of the polymer composition, and its molecular weight, provides a PAG product that is completely soluble in water at room temperature.
However, the selected PAG molecules exhibit the unique behavior of inverse solubility in water, that is, water insolubility at elevated temperatures. This phenomenon provides a unique mechanism for quenching by surrounding the metal piece with a polymer-rich layer that serves to govern the rate of heat extraction into the surrounding aqueous solution. As the metal part approaches the quenchant temperature, the PAG polymer coating dissolves to again provide a uniform concentration in the quenchant bath. In PAG quenchants, this occurs in the temperature range of 60-85°C depending on the specific polymer. See Figure 4.
This mechanism of inverse solubility is limited to two polymer quenchant classes: PAG and PEOX. In these systems, as the temperature of the solution is raised, the thermal energy of the system becomes greater than the energy of the hydrogen-bond interactions with water. When this occurs, a two-phase system develops, with one layer being water-rich and the other a polymer-rich layer (Figure 5). This is not a clean separation as both phases have some of the other components. The temperature at which this separation occurs is called the cloud point. In PAG quenchants, the ratio of the monomers used to produce PAG quenchants control the cloud point. In this case, the cloud temperature decreases as the propylene oxide monomer proportion increases.
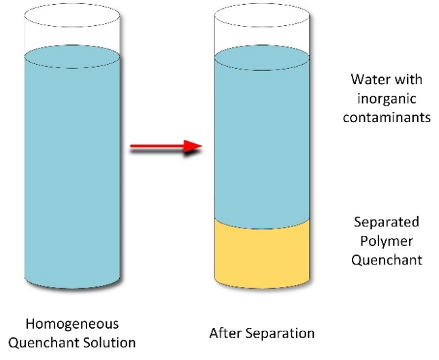
While thermal separation can be used to recover the polymer, the water-soluble corrosion inhibitors will report to the water phase. Once the polymer solution is reconstituted with water, additions of corrosion inhibitors must be added to the solution to maintain proper corrosion inhibition.
While corrosion can be an issue when quenching with water or aqueous salt solutions, solutions of PAG quenchants may be inhibited to provide corrosion protection of the quench-system components. Corrosion inhibition of quenched parts will be of short duration, so that specific protection should be provided following the tempering operation.
Effect of Concentration, Temperature, and Agitation
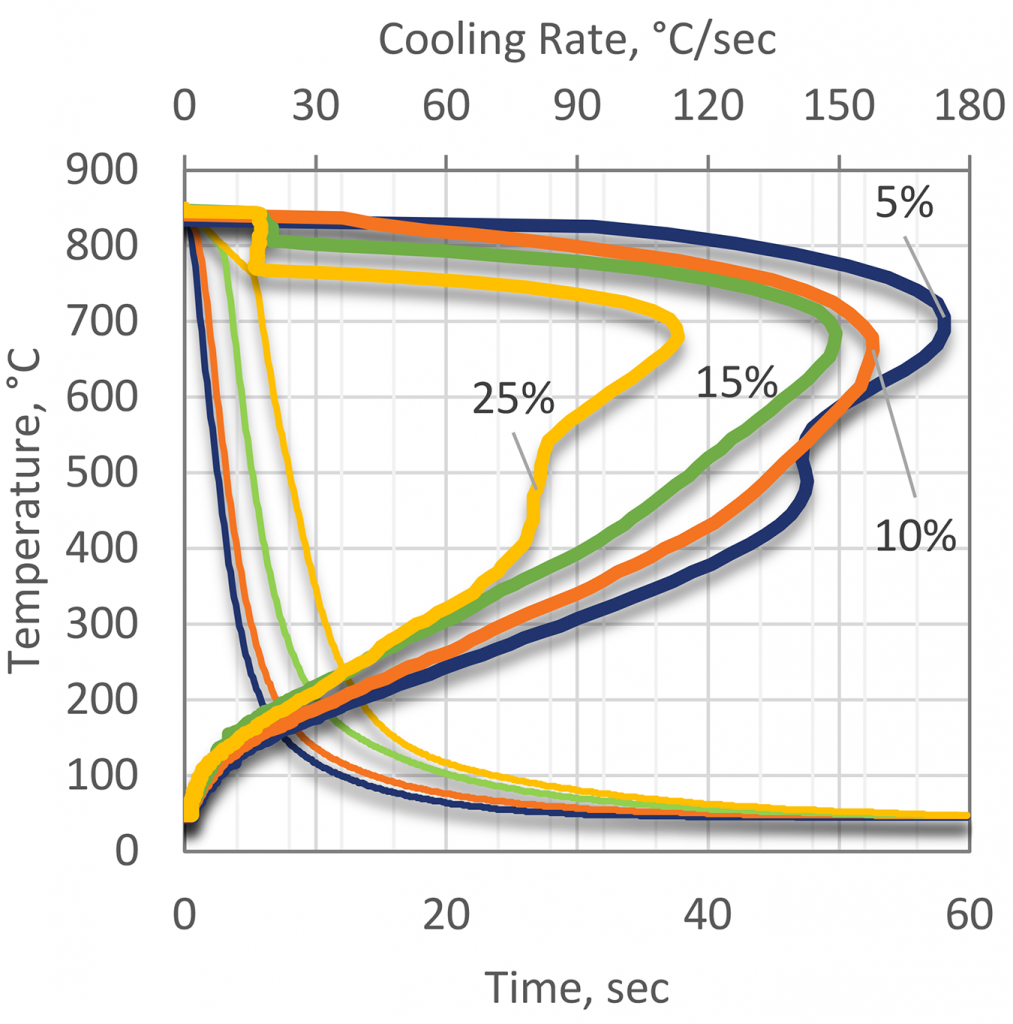
As concentration is increased, the overall rate of quenching decreases (Figure 6). This is due to the increasing thickness of the polymer film as the concentration increases.
Different PAG quenchants will exhibit different cooling curves, as it is dependent on the molecular weight of the polymer. As the molecular weight increases, the cooling rate decreases.
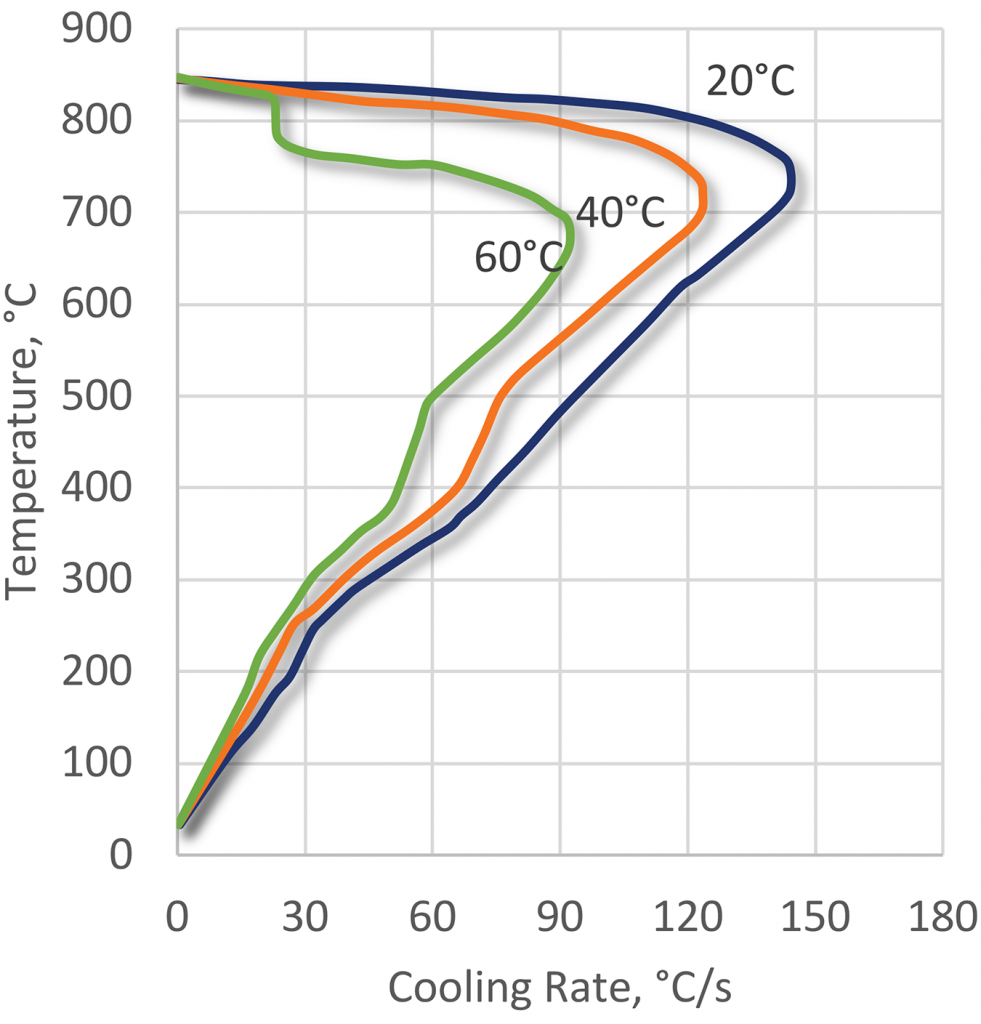
Just as water exhibits a loss in quenching power as temperature is raised, so do PAG quenchants. As the temperature is increased, the quenching power of PAG quenchants decreases (Figure 7). In general, PAG quenchants are limited in bulk temperature rise to less than 25°C less than their cloud point. If the bulk temperature exceeds the cloud-point temperature, then separation of the polymer could occur. If parts are extracted at temperatures above the cloud point, then excessive drag-out of the polymer would occur.
Agitation is always recommended for PAG polymer quenchants (or any other polymer quenchant). In general, low to moderate agitation is essential (a) to ensure that adequate replenishment of polymer occurs at the hot metal surface and (b) to provide uniform heat transfer from the hot part to the surrounding reservoir of cooler quenchant. Vigorous agitation may be essential for achieving a rapid rate of cooling (for example, with a low hardenability steel) to avoid undesired transformation.
Immersion quenching of steel components
The primary reason for consideration of PAG quenchants for steel quenching is the elimination of the smoke, fume, and fire hazards associated with quenching oils, although the benefits of flexibility of quenching speed and improved process economics are also important.
PAG quenchants are suitable for a wide range of steels, including plain carbon steels, boron steels, spring steels, general engineering steels, martensitic stainless steels, low- and medium-alloy carburizing steels, and higher-alloy steels of heavier section thickness.
Components that can be processed range from very small parts with section size as small as 1 mm (such as needles, circlips, screws, and fasteners) up to large shafts and forgings weighing 10 tons or more.
Between these extremes, a very wide range of components including bolts, bearings, crankshafts, springs, steel bars and coils, high-pressure gas cylinders, general forgings, and agricultural and automotive parts have been successfully quenched into PAGs.

Induction hardening applications of PAG quenchants
PAG quenchants are used widely for the quenching of components after induction or flame hardening as an alternative to water, soluble oil, or mineral oil. They are generally used at concentrations of 5-15 percent to eliminate spotty hardening associated with water quenching, to control distortion, and to provide corrosion protection to the induction hardening equipment.
Typical applications include the quenching of gears, crankshafts, camshafts, drive shafts, bearing rings, tubes, and bars (Figure 8 and Figure 9).
Quenching of aluminum alloys
PAG quenchants are used widely as an alternative to water or oil for the quenching of aluminum parts such as thin-section airframe and skin components, castings and extrusions for aerospace applications, and engine blocks, cylinder heads and wheels for the automotive industry.
Typically, these quenchants are governed by AMS 3025 [4], and are either Type I or Type II quenchants. Type I quenchants are single polyalkylene glycol polymers, while Type II quenchants are multiple molecular weight polyalkylene glycol polymers. Each offers different benefits. Because of the higher molecular weight of the Type II PAG quenchants, lower concentrations can be used.
The controlled uniform quenching characteristics of PAGs can significantly reduce or even eliminate the distortion often associated with water quenching without impairing mechanical properties or corrosion resistance. This is particularly important with thin-section sheet aluminum airframe components in the aerospace industry and complex castings and forgings, which are often quenched into boiling water or mineral oil to minimize distortion. [5]
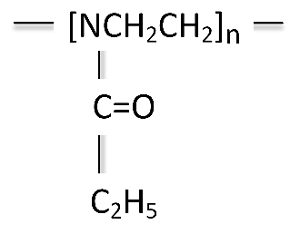
2 Polyethyl Oxazoline (PEOX)
This type of polymer has a wide applicability to low and high hardenability steels and different types of quenching.
Polyethyl oxazoline-based products represent the second generation of polymer quenchants. Developed as part of a continuing program of product innovation and improvement, PEO technology is now covered by worldwide patents.
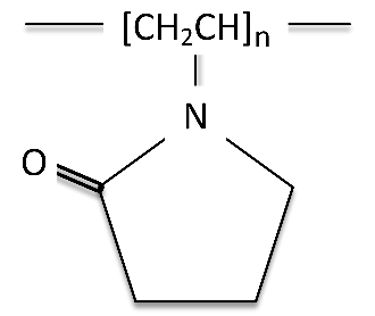
Polyethyl oxazoline (PEOX) is synthesized from the polymerization of ethyl oxazoline (Figure 10).
PEO-based products have similar characteristics of PAG quenchants commercially available and, as a result, are being used in a wide range of applications, ranging from induction hardening of steel and cast iron to tank quenching of high hardenability steel castings and forgings.
PEO chemistry is highly efficient in modifying quenching characteristics, and required properties are obtained at low polymer concentrations. This results in less drag-out than with other types of polymers and consequently great economy in use. Any polymer remaining on the surface dries to a hard, tack-free film, ensuring freedom from sticky residues.
Quenching Characteristics of PEOX Quenchants
PEO-based quenchants exhibit inverse solubility at a temperature of 60-65°C. Hence, the quenching mechanism is very similar to that of the polyalkylene glycol-based products. Like all other types of polymer quenchants, the quenching characteristics are dependent upon polymer concentration, bath temperature, and degree of agitation.
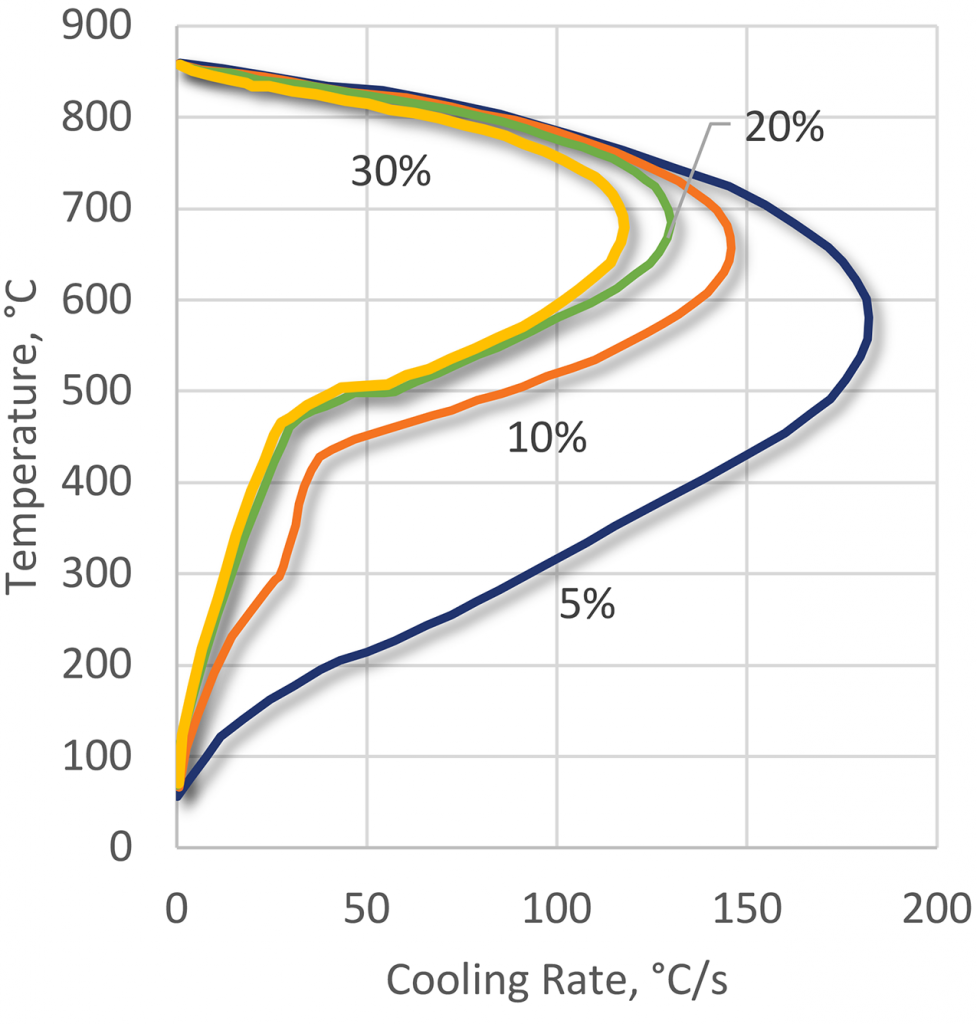
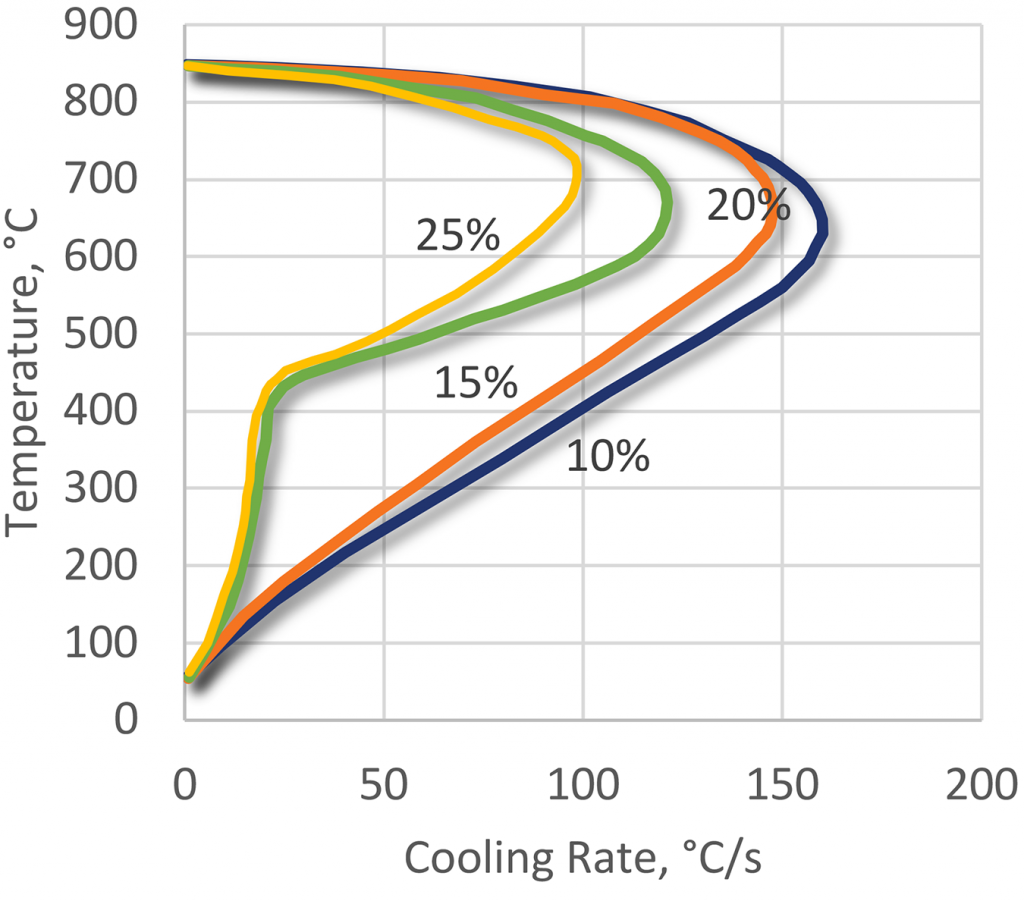
PEO polymers can be used over a wide range of concentrations from 5-25 percent (Figure 11) depending upon the application. PEO products have the least stable vapor phase of all the polymer quenchants and this is particularly important during induction hardening and in the quenching of low hardenability steels.
Also, the very low cooling rate in the convection phase, where, at concentrations of 15-25 percent, the quenching speed is almost identical to that of quenching oil. This extends the range of applications to critical high-alloy steels.
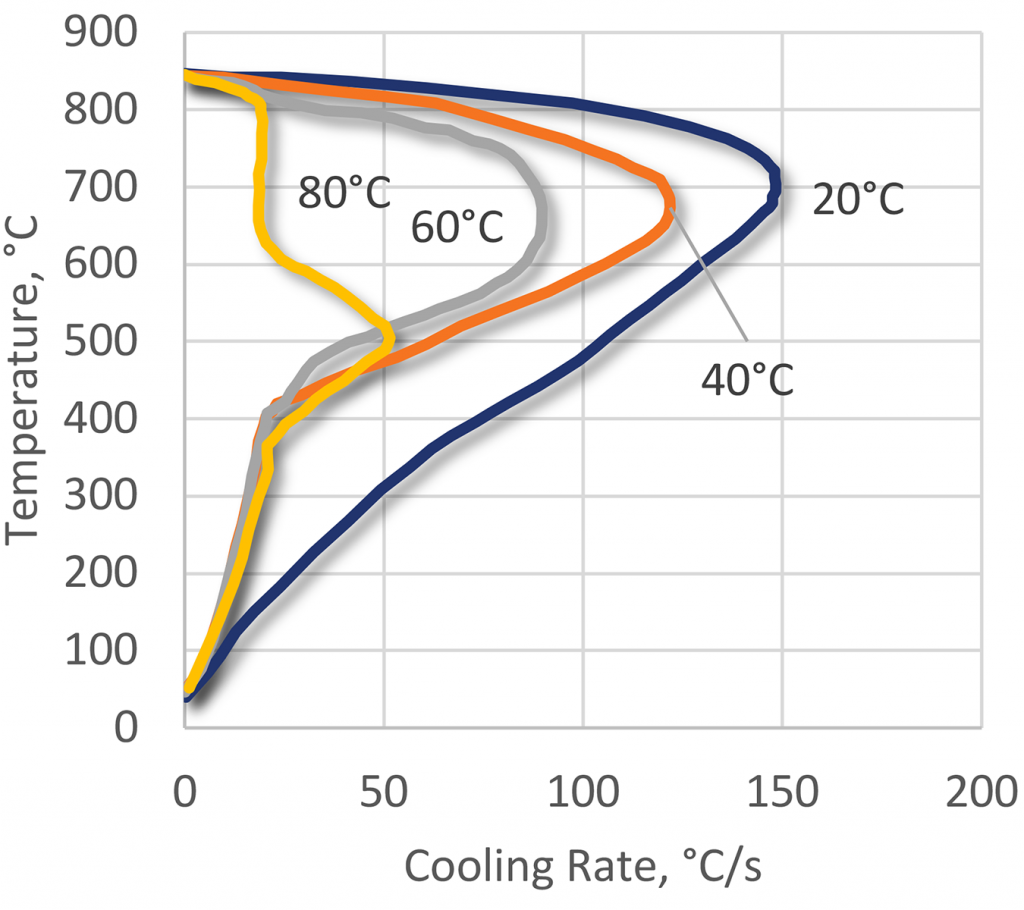
As with all polymer quenchants, as the temperature is increased, the effective cooling rate decreases.
Because PEOX products have an inverse solubility temperature of approximately 63°C, it is important to ensure effective cooling of the bath, and to limit the bulk quench temperature to 40°C or so.
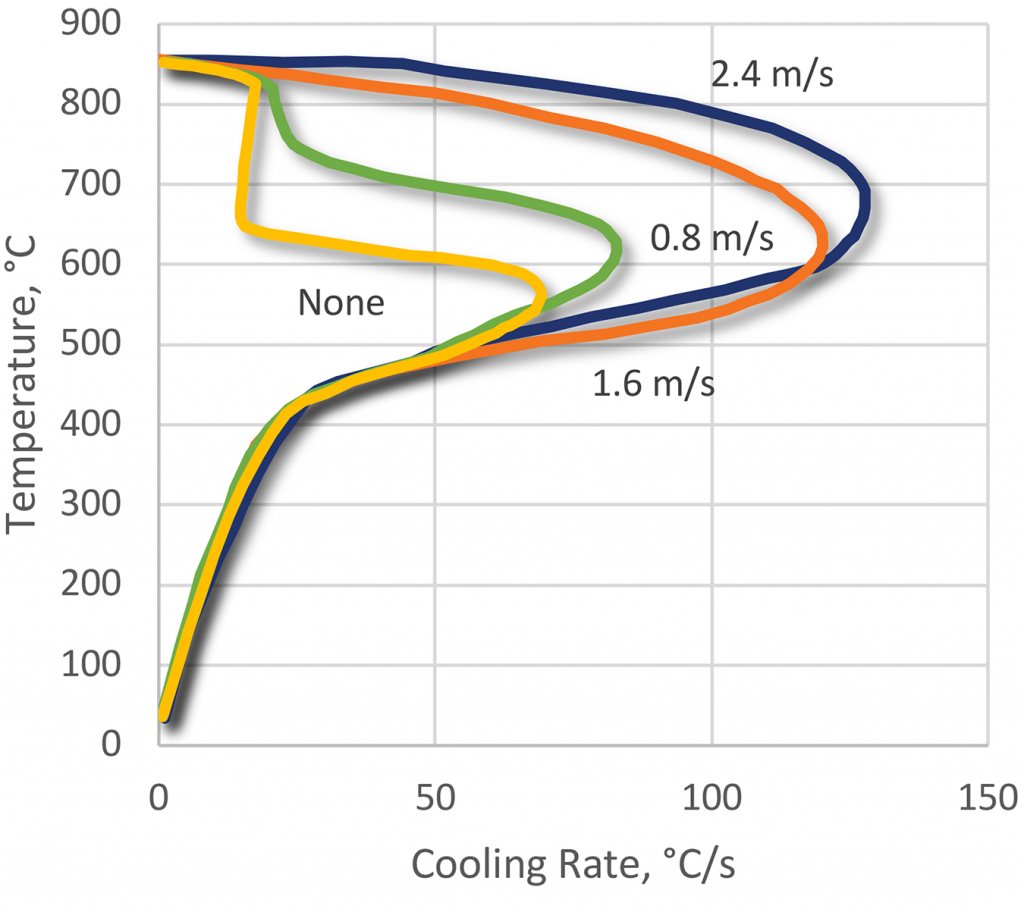
As with all polymer quenchants, the cooling effect is dependent upon the degree of agitation. However, the vapor stage with PEOX products is easily broken down and disappears completely with turbulent agitation. This is particularly important in the quenching of low hardenability materials.
As the agitation rate increases, the maximum cooling rate is increased, as with the temperature of maximum cooling.
Typical Applications for PEOX Quenchants
The flexibility of quenching speed, ease of breakdown of the vapor phase, low cooling rate in the convection stage, and freedom from sticky deposits enable the use of PEO-based products for a very wide range of applications.
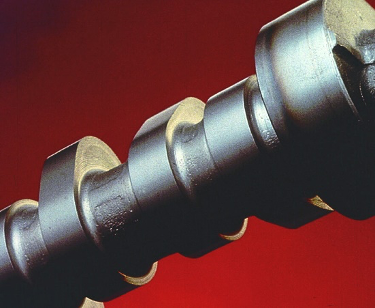
Induction and flame hardening: Generally used at concentrations of 5-10 percent as a replacement for PAGs or oil in the treatment of steel and nodular cast-iron components. Typical applications include camshafts (Figure 12), crankshafts, and gears in the automotive industry, and drill pipe for the oil industry. The hard, dry residual film is of particular benefit to subsequent handling operations or where shot-blasting is required.
Quenching of low hardenability steels: The short vapor phase enables the development of maximum properties in low hardenability materials such as those used for fasteners; 10 percent PEO solutions are used successfully for the treatment of bolts and screws in continuous furnaces. Good agitation is required to minimize soft spots in these difficult-to-quench components.
Alloy steel forgings, bars, and castings: Low cooling rates in the convection phase make PEO polymers suitable for higher hardenability alloy steel components that would normally be oil quenched. (Figure 13) Concentrations range from 15 to 25 percent, depending upon applications that include martensitic stainless-steel wire and rod AISI 4100 and 4300 series castings and forgings. High carbon-chromium grinding balls and liner plates and nodular and ductile iron castings have all been successfully quenched in PEOX quenchants.
Other applications: PEOX quenchants have been used in continuous mesh belt type furnaces for quenching small bearing races, as well as smaller link chain applications. The benefits of low residue and high-cloud temperature result in a low drag-out product that can be readily washed off using cold or ambient temperature water.
While recovery of polymer is possible from rinse tanks by taking advantage of the inverse solubility and cloud point of PEOX polymers, the low residue and drag-out of PEOX polymers makes this possibly not economically feasible.
This type of polymer has a wide applicability to low- and high-hardenability steels and different types of quenching. Spray and immersion type quenching is possible. It has applications from continuous type furnaces to large quench tanks quenching large forgings and castings.
3 Polyvinyl Pyrrolidone (PVP)
Polyvinyl pyrrolidone (PVP) is derived from the polymerization of N-vinyl-2-pyrrolidone [6] (Figure 14). Polyvinyl pyrrolidone is a water-soluble polymer characterized by its unusual physical and colloidal properties and by its physiological inertness [7] [8].
Polyvinyl pyrrolidone has been available in the United States as a white, free-flowing powder manufactured in multiple grades ranging from low to high molecular weight.
The low molecular weight polymers have narrower distribution curves of molecular entities than the high molecular weight compounds.
PVP polymers are widely used in inks, adhesives, detergents, and soaps, as well as being an effective quenchant.

Quenching Characteristics of PVP
As with other polymer-type quenchants, concentration, bath temperature, and agitation all play a role in establishing the cooling characteristics. By comparison, the quenching rates tend to be faster during the stable film and nucleate and boiling stages but are slower during the convection stage. Because PVP does not have inverse solubility in water, only very small amounts of polymer film are retained on quenched parts at quenching temperatures from 30°C (85°F) to near boiling. Thus, a broader working range of temperatures for quenching can be employed.
The effect of concentration is shown in Figure 15. PVP-based polymers are generally used at concentrations of 10 to 25 percent where the quenching characteristics are very similar to quenching oils.

At higher concentrations, the cooling rate at 300°C is substantially reduced. This makes the quenchant extremely suitable for high hardenability forgings or other crack-prone components. The fast maximum cooling rate at lower concentrations makes it effective in hardening low hardenability parts, while minimizing the formation of non-martensitic transformation products (NMTP).
As the temperature of PVP solutions increases, the vapor phase is extended, and the maximum rate of cooling is reduced (Figure 16). At temperatures above 40°C, the cooling rate is decreased at 300°C, making it suitable for high hardenability alloys. Interrupted quenching is also beneficial.
A maximum operating temperature of 60°C is normally recommended to minimize evaporation losses from the system.
As with other polymer quenchants, the overall heat extraction increases as the agitation is increased. The vapor phase of PVP quenchants are less stable than other quenchants. However, it is still important to maintain good agitation. (Figure 17)
Typical Applications of PVP quenchants
The oil-like quenching characteristics of PVP polymers extend the applications to the heat treatment of high hardenability materials and alloys.
PVP-based products are used widely in the steel industry for the quenching of bars, rolled sections, and forgings, generally at concentrations of 10-25 percent.
The most common application of PVP quenchants is their application in quenching large forgings and castings. Quenching high hardenability rolled rings is a very common application. For high hardenability rolled rings, the concentration is typically about 10-15 percent, depending on the temperature and uniformity of agitation.
The low solids content and low residual film resulting from use of polyvinyl pyrrolidone-based quenchants is very conducive to the use of interrupted quenching or timed quenching. This allows very high carbon, or very crack-sensitive parts to be quenched effectively, while maintaining properties.
Polyvinyl pyrrolidone-based quenchants have also been used successfully in quenching of high hardenability rolled rings at concentrations of 10-12 percent. PVP quenchants are very important to control residual stresses and yet still achieve hardness in large forgings (Figure 18) and in die steels (Figure 19). This quenchant is being used worldwide to quench large steam turbines, as well as high nickel super alloy forgings used in aerospace for jet engines for turbine hubs. Large 3,000 kilogram high hardenability castings for the mining industry are commonly quenched in PVP. The concentration is typically 18-22 percent.
Other applications include induction hardening or replacement for oil in continuous-type furnaces, such as mesh-belt furnaces, for processing links and rollers in link chain, or small bearing components.
Polyvinyl pyrrolidone-based quenchants have been proven to be an effective replacement for oil for immersion quenching large forgings, as well as applications in continuous mesh-belt furnaces for quenching fasteners, bearing components, and link chain parts. The slow quenching characteristics compared to other quenchants at the same concentration often make this a suitable replacement for fast to medium speed oils.
4 Sodium Polyacrylate (ACR)
Sodium polyacrylate-based polymer quenchants have oil-like quenching characteristics, which enable the treatment of a wide range of alloy steels and higher hardenability materials.
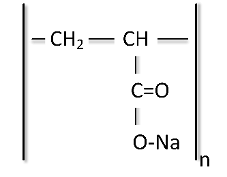
Sodium polyacrylate (ACR) quenchants are synthesized from a sodium acrylate monomer, with the chemical formula shown in Figure 20. This polymer is a sodium salt of polyacrylic acid and is used extensively in consumer products. By using the salt of an alkali metal, in this instance, sodium, the polymer provides solubility in water.
Polyacrylate quenchants represent a class of quenchants completely different from the PAG, PEOX, or PVP types. The latter polymers fall into the type characterized as nonionic, that is, not ionizable or neutral. Polyacrylate quenchants are considered anionic, which is negatively charged. The charged character of the polymer on parts adds another dimension to the quenchant: strong polarity. The strong polarity provides water solubility but also is suspected of causing the polymer to operate by a different mechanism of heat extraction. Unlike other polymer quenchants, polyacrylate solutions do not split on heating and do not form thick films on the surface of the hot work. Their slower rate of cooling is based on the molecular weight of the polyacrylate and the subsequent viscosity of its solutions. By varying the molecular weights of the polymer and the polymer concentration, a whole family of quenchants can be designed covering a full range of applications, from the fast quenching of water to the slow cooling of oils.
Quenching Characteristics of Sodium Polyacrylate Quenchants
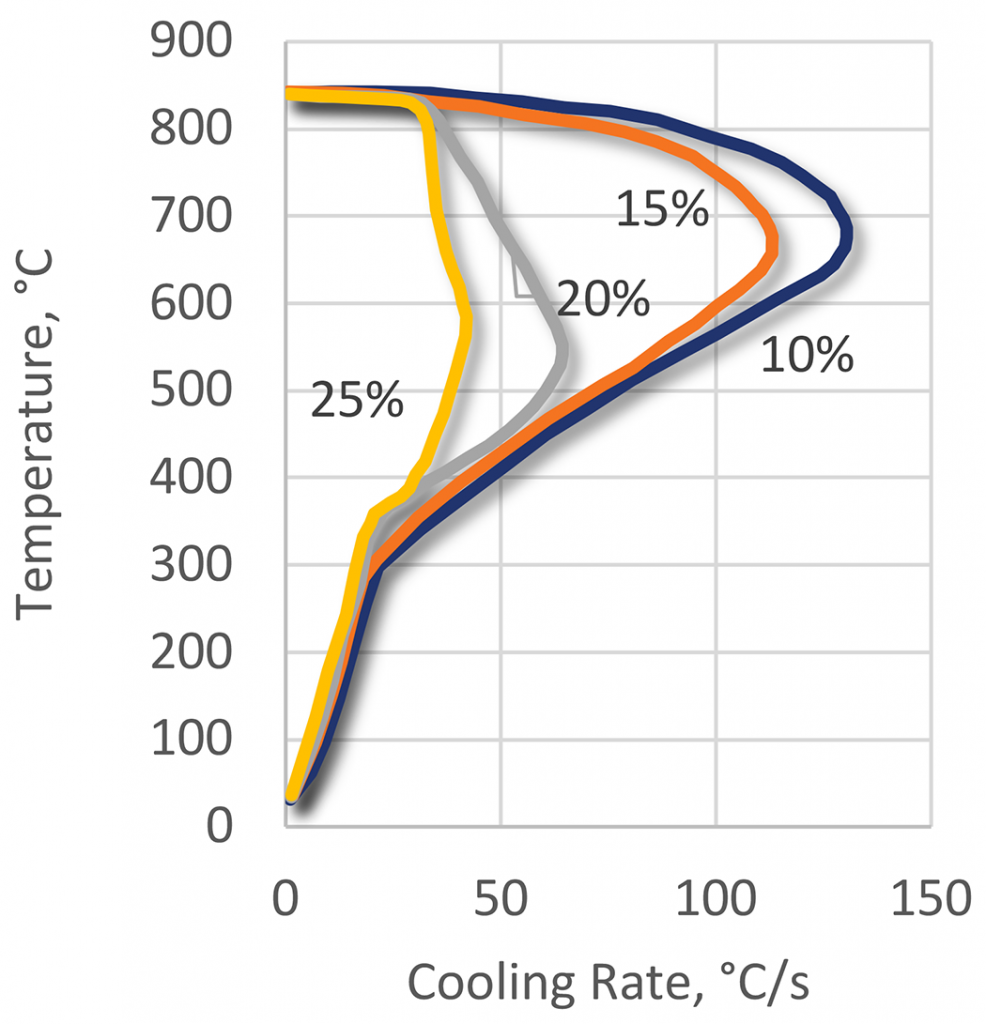
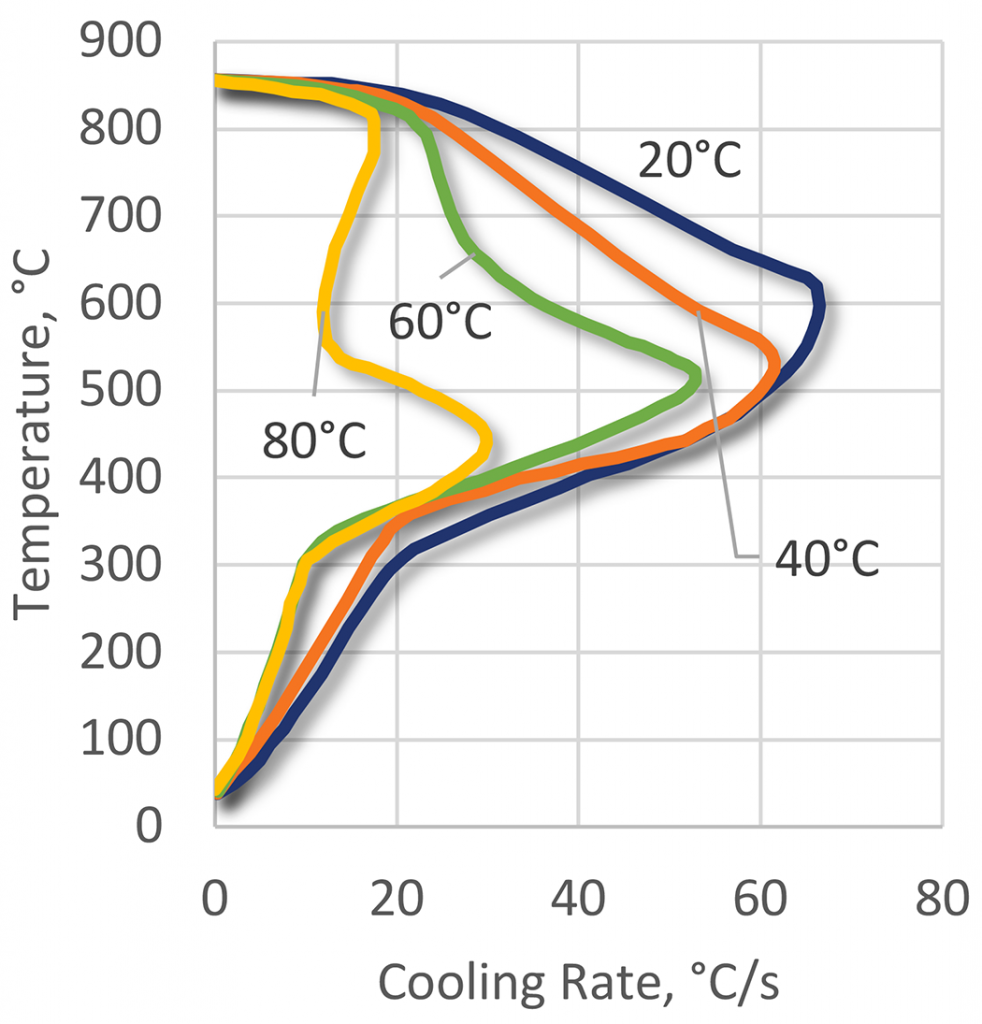
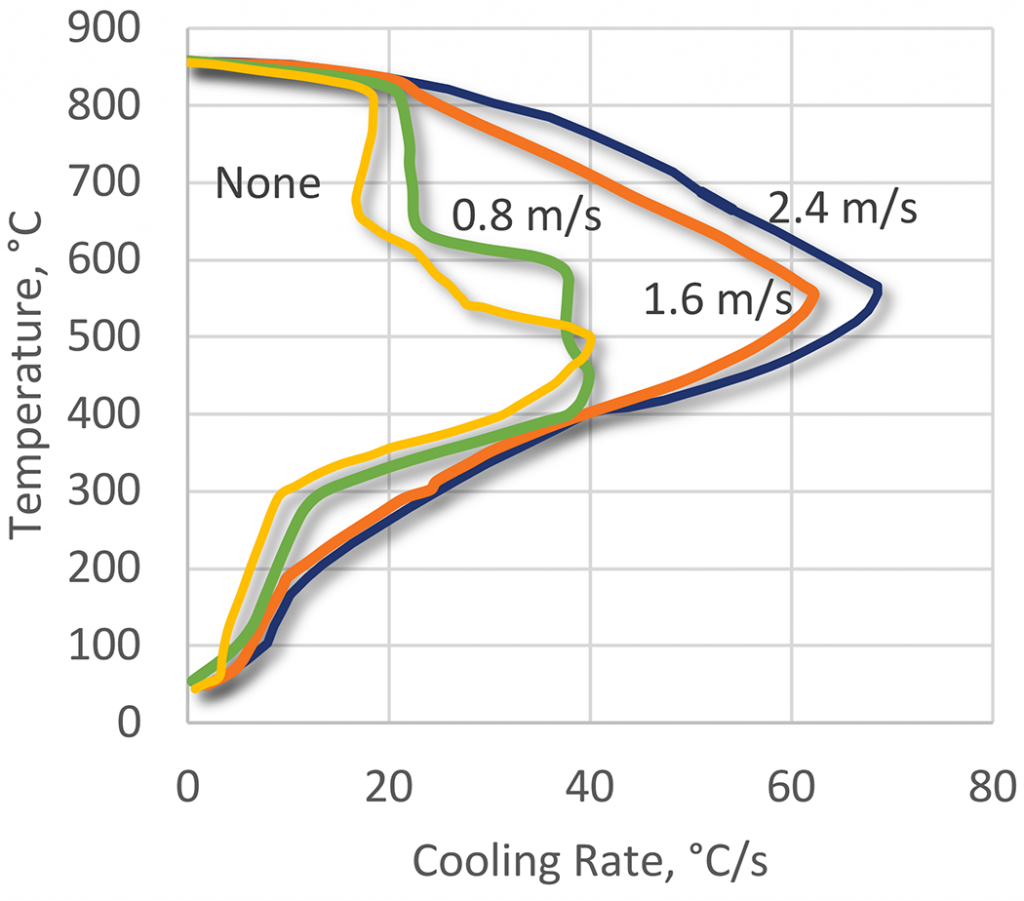
The quenching effect of the polyacrylate quenchants is a function of the three basic parameters: polymer concentration (Figure 21), bath temperature (Figure 22), and bath agitation (Figure 23). The cooling curves of the polyacrylate solutions can have an extended vapor phase and reduced heat extraction during the boiling phase. This polymer quenchant also has a very slow quench rate through the martensitic transformation region [11]. This unique property of the polyacrylate quenchants allows their applications for hardening of crack-prone parts made of high-hardenability steels. Applications of this kind usually are unobtainable with any other polymer quenchants or require much higher concentrations of the polymer.
The quenching characteristics of ACR solutions are sensitive to agitation as shown in Figure 23. Under static conditions, ACR solutions exhibit a pronounced vapor phase. As the severity of agitation increases, the vapor phase is shortened, and there is a significant increase in maximum cooling rate. As with other polymer quenchants, agitation is important to achieve uniform quenching. Generally, a high degree of agitation is recommended for hardening operations because of the persistent vapor phase.
The drag-out of sodium polyacrylate is much higher than either PAG or PVP quenchants at the same concentration. It has been reported that the drag-out of ACR quenchants have a drag-out of twice PAG-type quenchants, and four times that of PVP quenchants [12].

Typical Applications
The oil-like quenching characteristics of ACR polymers enable the heat treatment of higher hardenability materials. While most applications are for immersion quenching, there have been a few unique applications (Figures 24 and 25). These include critical AISI 4140 seamless tubes for the oil industry, AISI 4140 and 4340 forgings and castings, heavy gears, thin-section alloy steel crankshafts, and high-carbon chromium grinding balls. Another application is for thick section gas cylinder applications. It is also used as a replacement for molten lead baths in wire patenting.
Concentration control of sodium polyacrylates is based on the kinematic viscosities. However, as solutions age, they tend to shear. One of the major control issues is that the sodium may ionize in solution and become sensitive to hard water, forming soluble carboxylic salts. This results in a change in viscosity, which makes concentration control more difficult. The effective quench rate is then based on viscosity, refractive index, and effective cooling rate. Because of the problems with process control of concentration, the application of ACR polymers has a limited market share to specialized operations such as quenching of white iron grinding balls, patenting of wire and quenching of high hardenability forgings.
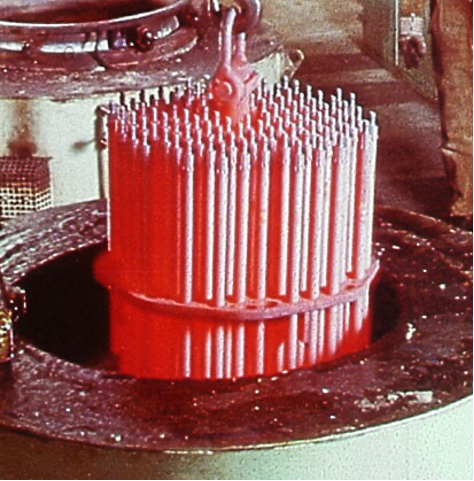
The sodium polyacrylate polymer quenchants are characterized by a very long and stable vapor phase, with a very slow cooling rate through the martensitic transformation rate. This quenchant has been used for quenching of very high hardenability forgings and used for the patenting of steel wire. At the same concentration, sodium polyacrylate quenchants have a much slower overall quench rate than either the PAG or PVP-type quenchants.
References
- ASTM International, “Standard Test Method for Determination of Cooling Characteristics of Aqueous Polymer Quenchants by Cooling Curve Analysis with Agitation (Tensi Method),” ASTM International, Conshohocken, PA, 2016.
- Houghton International, Inc., “Houghton on Quenching,” 1991.
- G. Totten, C. Bates and N. Clinton, Eds., Handbook of Quenching and Quenchants, Metals Park, OH: ASM International, 1993.
- SAE, “AMS 3025E Polyalkalene Glycol Heat Treat Quenchant,” SAE, Warren, PA, 2018.
- P. M. Kavalco, L. C. Canale and G. E. Totten, “Distortion Reduction by Aqueous Polymer Quenching of Aluminum Alloys,” Industrial Heating, vol. 2, no. February, p. 39, 2011.
- F. Haaf, A. Sanner and F. Straub, “Polymers of N-Vinylpyrrolidone: Synthesis, Characterization and Uses,” Polymer Journal, vol. 17, pp. 143-152, 1985.
- B. Liscic, H. M. Tensi, L. C. Canale and G. E. Totten, Eds., Quenching Theory and Technology, Boca Raton, FL: CRC Press, 2010.
- G. Totten, C. Bates and N. Clinton, Eds., Handbook of Quenching and Quenchants, Metals Park, OH: ASM International, 1993.
- P. Cary, Quenching and Control of Distortion, Metals Park, OH: ASM International, 1988.
- D. S. MacKenzie, “Quenchants Used for Heat Treating of Ferrous Alloys,” in Steel Heat Treating Technologies, vol. 4B, J. L. Dossett and G. E. Totten, Eds., Materials Park, OH: ASM International, 2014, pp. 255-280.
- F. F. Griffiths, The Quenching Characteristics of Sodium Polyacrylate Solutions – Ph.D. Dissertation, Sheffield, UK: Dept. Metals and Materials Engineering, Sheffield City Polytechnic, 1989.
- Edgar Vaughn & Company, “Quenching Principles and Practice”, Manchester, UK: Edgar Vaughn & Company, 1885.