
In this column, we will consider safety aspects of quenching with oil or molten salt.
Heat treating is a manufacturing process where the goal is to change the microstructural characteristics of the metal being treated. At its simplest, for steel parts, this is usually accomplished by heating to above the austenite temperature, quenching in a suitable quenchant to harden the part into martensite, followed by tempering to reduce residual stresses, and turn the hard and brittle martensite to more ductile, tempered martensite.
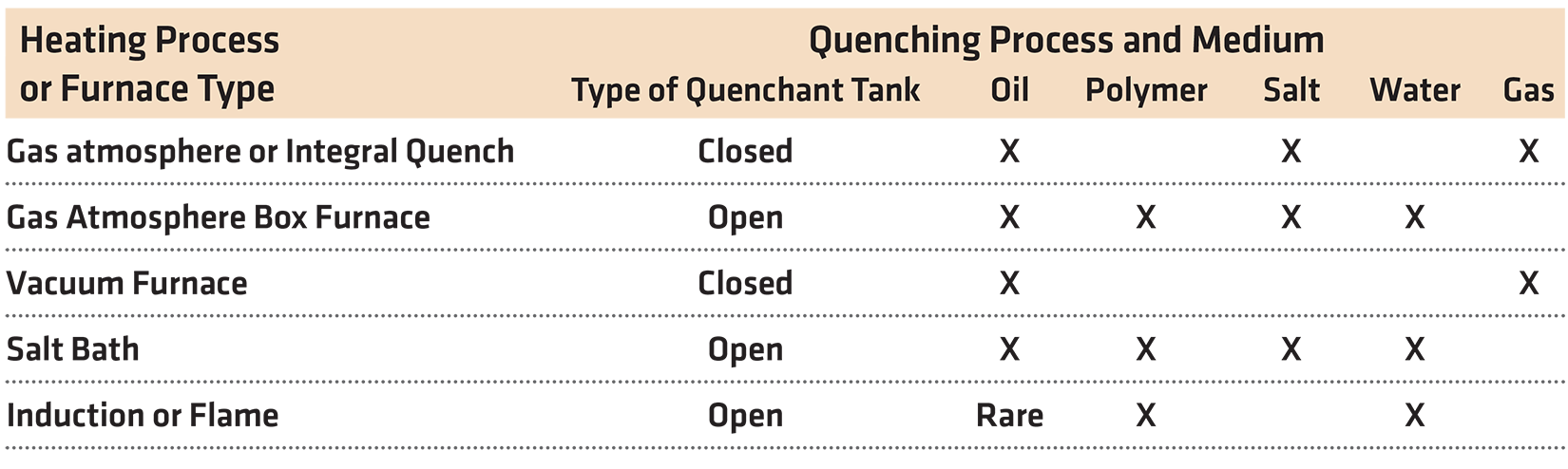
To heat the part to the austenite region, there are four general methods of accomplishing this. The most common method is to heat the parts using either gas or electric in a gas atmosphere. Parts can be heated under a vacuum to reduce surface oxidation. Parts can also be heated in a molten salt bath, but this is becoming less common due to health, safety, and environmental concerns. Finally, parts can be heated completely, or at the surface using induction or flame. There are also many other ways to locally heat a part, but that is beyond the scope of this article.
For quenching, and converting the austenite to martensite, there are really two methods. The first is by either immersing, spraying, or flooding the part with liquid quenchant. The second method is by rapid-cooling the part using gas (air, nitrogen, helium, argon, or mixtures, etc.). Again, the goal of quenching is to convert the austenite to hard martensite using a controlled cooling process.
Not every heating process is compatible with the different quenchants. For instance, the workhorse sealed integral quench furnace, is not suitable for polymer or water-based quenchants. This is because the water vapor from the quench tank can infiltrate the hot zone and contaminate the atmosphere. Expensive modifications are required to allow water or polymer quenchants in integral quench furnaces. Oil (most common), molten salt, and high-pressure gas have been used as quenchants for sealed quench type furnaces.
Induction or flame hardening using oil as the quenchant is rare, but it is done commercially. This heating process is usually followed by a water or polymer quench, the idea being to reduce or eliminate fire hazards.
A simple table showing the various combinations of heating process and furnace types, with their associated compatible quenchants, is shown in Table 1.
Fire and Explosion Hazards
Furnace Atmospheres
A typical furnace atmosphere used for heat treating steel parts is composed of roughly 20% CO, 0.5% CO2, 40% H2, trace water vapor, and the balance N2. At temperatures below approximately 750°C, this atmosphere is explosive. These explosive conditions can occur when loading or unloading parts, allowing oxygen to enter the work zone. The flame curtain or heating elements can act as the ignition source. A leaky furnace, or a furnace with an internal negative pressure can also allow air to infiltrate. It is imperative that a suitable flame curtain and process controls be implemented and maintained properly for atmosphere operation. Operators should be properly trained concerning the hazards associated with the heat-treating operation.

Oil Quenching
Fire hazards accompany oil quenching. Hot parts and flammable oil can lead to some pretty spectacular fires if proper controls are not implemented.
Water in the quench tank can contribute to a very hazardous situation during quenching. Water expands up to 1,600 times when turning to steam. This can result in a rather large fire and explosion in a sealed quench furnace, or a froth over in open quench tanks. Keeping any water content of the quench oil below 1,000 ppm will reduce the chance of a water-induced oil fire.
Hung loads, or parts partially submerged in a quench tank, can cause a large fire. The oil at the surface will locally overheat and form combustible vapors that can be ignited by the partially submerged part. Some engineering controls used to combat this are rapid dumps of the quench oil to a reservoir away from the parts, or a rapid release to allow the part to be rapidly submerged in the quench.
Improper oil level (either too high or too low) can result in oil fires. In the case of too low an oil level, the oil inadequately covers the part, resulting in an overheated surface layer, like a hung load. The quantity of oil present could exceed the flash point, and “flash off,” resulting in a large oil fire. Excessive oil levels can result in the oil expanding and reaching the hot zone (in an integral quench furnace), or overflow in an open quench tank.
Finally, the ventilation used to exhaust oil quench fumes from the heat-treat shop can become saturated with condensed oil. These types of fires can occur if the flames from the integral quench furnace flame curtain or the exhaust over the burn-off catch the condensed oil on fire. The fire can rapidly spread and be difficult to contain. Maintenance to routinely clean the ductwork should be implemented. The use of higher flashpoint quench oils can reduce the quantity of fumes, which reduces the amount of condensation in the ductwork. Finally, a method of extinguishing ventilation fires, such as flooding with nitrogen or fire extinguishers, should be part of facility design.
 Molten Salt Quenching
Molten Salt Quenching
Quenching in molten salt offers many benefits, including reduced distortion, and the ability to austemper suitable steel parts. However, many of the beneficial characteristics of salt baths can make fires difficult to contain and extinguish.
Salts used for quenching are usually operated at below 350°C and are predominantly mixtures of sodium and potassium nitrates/nitrites. Molten salts used for austenitizing are mixtures of sodium and potassium chlorides. High-temperature carburizing salts can contain cyanide.
Nitrate/nitrite molten salt baths that are operated either intentionally or accidentally above 650°C can result in a large explosion. Nitrate/nitrite molten salt quench tanks (or heating as in the case of aluminum) are prohibited from being operated above 650°C. Operating controls such as high limit controllers and process controllers should be set to shut down the heaters should it detect the thermocouple is open.
Quenching from cyanide carburizing baths into nitrate baths will result in a severe exothermic reaction and the release of cyanide gas. Acids should never be stored near drums of cyanide-containing salt.
Organic materials on parts, or baskets and fixtures will burn. Parts and baskets should be thoroughly clean prior to entering the salt bath. This prevents fires and ensures a nice stain-free surface when exiting the bath.
If a molten salt quench tank (or a high heat austenitizing molten salt bath) is allowed to cool, the molten salt will solidify. If allowed to sit, water can sometimes infiltrate the quench tank. Because the solidified tank is opaque, it is difficult to see any water that is at the bottom of the tank or entrained in pockets. It is critical, prior to heating the molten salt, that multiple pathways be created to allow superheated steam in the tank to escape. Otherwise, a large steam explosion can occur. Gradually heating the salt with a torch until the salt is molten enough to be heated by the heating elements or radiant tube is also good practice.
Fighting a salt fire is not straight-forward. Direct streams of water should not be directed at the molten salt. The water can get under the molten salt, and result in a large steam explosion, with the molten salt coming at you. As a young engineer, I had a rather large salt bath fire in my shop. As the firemen came rushing in, I had to stand in front of the hose to prevent the fire first responders from pointing a high-pressure water hose at the flaming salt bath. Had they been successful in pointing the hose at the fire, there would have been multiple injuries and a large explosion.
It is important to relay to the first responders the potential hazards that they might face. This is to ensure that the situation doesn’t go from bad to worse.
Conclusions
Heat treating can be hazardous if the necessary precautions are not implemented and followed. Engineering controls must be in place, and people must be properly trained to operate equipment safely. They should be trained how to respond to upsets in the process. Finally, first responders should be notified of the hazards that they may face when dealing with a fire.
Should there be any questions or comments on this article, or suggestions for further articles, please contact the writer or editor.

























