With the COVID-19 pandemic still raging across the country and the world, the need for personal protective equipment for medical professionals has pushed the production and distribution of this literal life-saving necessity to their limits.
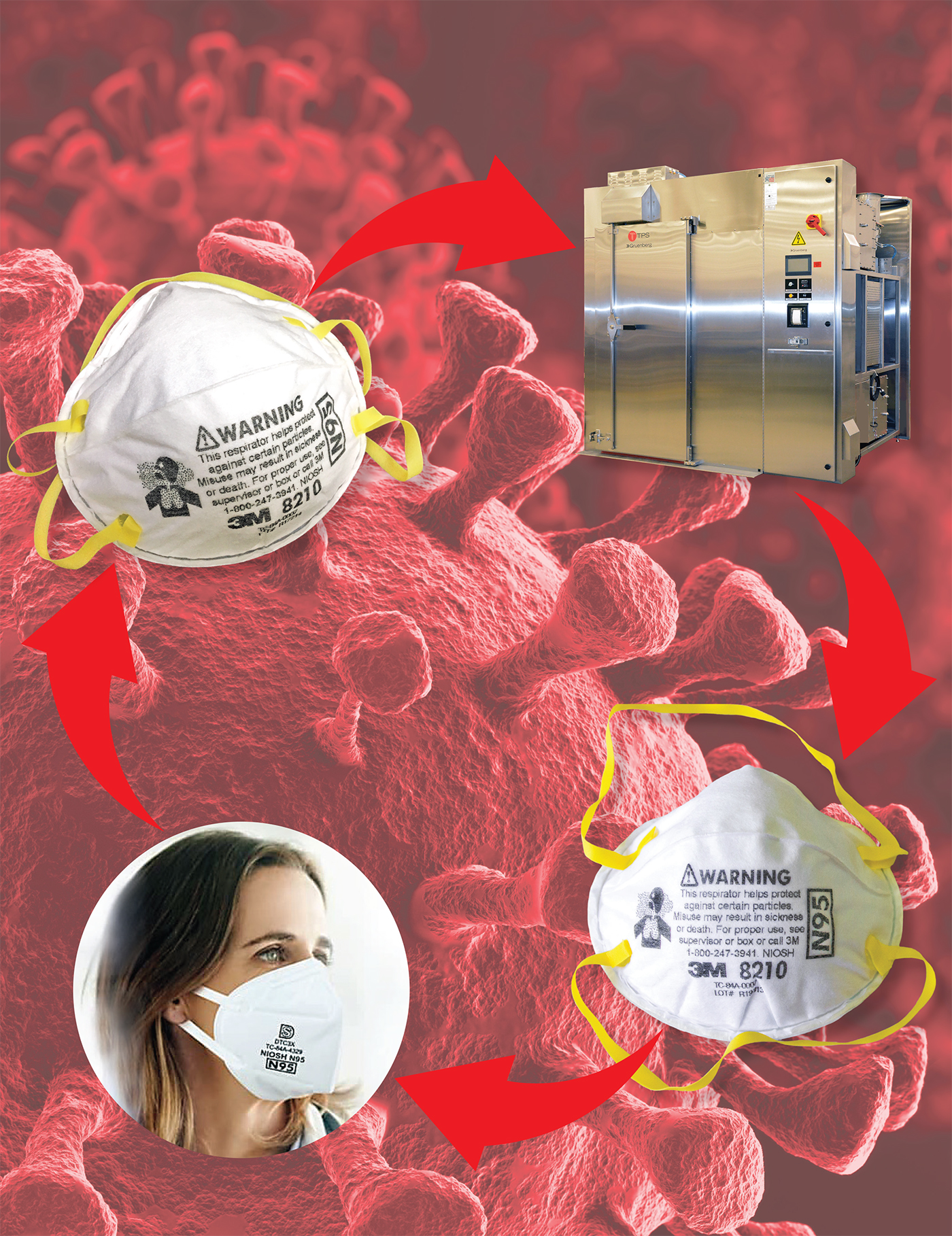
Given the ongoing challenge of demand exceeding production, Thermal Product Solutions (TPS) has been working with health professionals to use the company’s Gruenberg product line for dry heat sterilization to treat PPE and N95 masks for re-use. The Gruenberg sterilizers use convection airflow and dry heat for the process.
In order to evaluate the impact of dry heat treatment on 3M respirator masks, TPS teamed with Stony Brook Medicine, Long Island’s premier academic medical center.
So far, the results have been extremely promising.
The experiment begins
Using a Gruenberg sterilizer to disinfect PPE was part of an initiative started by Stony Brook University’s Dr. Kenneth R. Shroyer. The Gruenberg dry heat sterilizer was recently installed in a lab animal cage wash facility and had just started acceptance testing within the hospital when the researchers reached out to Robert Davis with TPS’s representative, Process Control Solutions, to help refine their process.
“We were waiting for this large VHP equipment to come from Battelle Memorial, which it ultimately did arrive,” said Glen Itzkowitz, Associate Dean for Facilities and Operations at Stony Brook. “Prior to the installation of the Battelle vaporized hydrogen peroxide system, there was an effort on the ground to try to disinfect model 1860 masks, a rigid frame N95 mask, by heat.”
VHP, or vaporized hydrogen peroxide, is a recently approved method for sterilizing some pieces of PPE for reuse.
First efforts to sterilize the masks and PPE using traditional autoclaving methods were unsuccessful, which is where TPS’s Davis entered the picture.
“They were having some questions about their cycles that we had already validated for their laboratory, and they called me to ask some follow-up questions,” Davis said. “Then they started to say that they had been sterilizing N95 masks in their dry heat sterilizer but had some questions and needed some additional validation help.”
Autoclave dilemma
Hospitals typically use an autoclave to sterilize equipment using steam heated to 121°C, which is why that initial temperature was used for the dry heat test, according to Davis.
The problem with using an autoclave for PPE and N95 mask sterilization is that the autoclave process distorts the masks and causes problems with fitting.
“The fit test fails,” Davis said. “The fit test includes how it fits around your face and seals, but it also looks at the filtration efficiency of the mask.”
With autoclave attempts not working and with Stony Brook still waiting on the VHP equipment, Itzkowitz said Plan B was to try the new Gruenberg dry heat sterilizer in the facility’s Medical and Research Translational Science Building (MART). Stony Brook first learned about dry heat sterilization from the Dana Farber Cancer Center, where it is used in its lab animal facility.
“They use quite a bit of this technology,” he said. “The concept was good for us because this was in a cancer center research building as well. So, we had this brand new TPS Gruenberg dry heat sterilizer that was installed, and we were in the process of just starting to validate it for lab animal work when we shut down due to COVID-19. We basically put our research programs on a 10,000-foot holding pattern.”
That’s when Stony Brook’s dry heat sterilizer came into play, according to Davis.
Performing tests
With significant scientific input from two pre-doctoral students from Dr. Shroyer’s lab, Stony Brook successfully completed a series of rigorous tests. Initially, three tests were performed on 3M 1860 and 1870 masks. For safety’s sake, actual COVID-19 wasn’t used in the tests.
A lot is known about Coronaviruses in general, and although COVID-19 is a strain of Corona, the thermal destructive range of COVID-19 has yet to be verified, according to Itzkowitz.
“Coronavirus has been in the veterinarian community as a canine health concern for a long time,” he said. “Someone’s going to eventually publish a thermal destruction number for dry heat disinfection of SARS CoV2/COVID-19 Novel Coronavirus.”
In one of the tests, masks were inoculated with surrogate bacterial markers, placed in a lightweight paper bag, and sterilized in the Gruenberg dry heat sterilizer at 100°C for 30 minutes, according to Davis. The masks were cultured after dry heat disinfection and were found to have no presence of inoculated bacteria.
Assistance from TPS
During the testing, one of the main assists from TPS was to install temperature probes to aid in the process, according to Itzkowitz.
“We got really good information about where the cooler spots and the hotter spots were in the dry heat sterilizer, so we could ensure that we were truly creating an apples-to-apples test,” he said. “That was invaluable learning to us. Now that we understood that, we went back and started adjusting the recipes accordingly.”
There is a certain temperature and a certain time at temperature that is required, according to Jeff Kent, director of sales for TPS.
“Depending upon how dense the product is loaded and depending upon what the actual product is, that’s all going to affect the heat-up time,” he said. “So even if you’re destroying the same pathogen, you can have a wide variety of total cycle times.”
Even with those variables, during the testing, Stony Brook proved the PPE could be treated at 100°C, which should be sufficient to disinfect COVID-19, according to Kent.
The reason Stony Brook initially chose 121°C is it’s the temperature that autoclaves use, according to Davis.
“Subsequent to that, there was a little bit of information out there saying these masks could be sterilized effectively at lower temperatures,” he said. “Then they did a more extensive follow-up test showing that these could be treated at 100°C, and that’s the data that they ultimately put out to some pathology associations.”
Surprising results
After some discussion, Stony Brook conducted another round of tests, specifically on the model 1860 masks, according to Davis. Masks were placed in light weight brown paper bags, labeled for single blind quantitative testing, and incubated for 30 minutes at 100˚C. Fit tests were performed after one cycle and again after four total cycles of heat treatment using a respirator fit tester to measure the 300 nm particle concentration outside and inside the mask to calculate fit factor to OSHA (US)-compliant fit test methods. It was concluded that the dry heat treatment at 100˚C could be used to enable re-use of either model 1860 or 1870 N95 masks. The final test scores show the sterilized masks could be re-used through quantitative testing, according to Itzkowitz.
“What we believe is that at temperatures below the thermally destructive threshold of the mask itself, we could probably dry heat treat these masks many more times than we did,” he said. “These temperatures did not structurally degrade the masks or negatively impact their integrity to filter air, as based on the data of the quantitative fit testing — which is great.”
Theoretically, according to Itzkowitz, for a small community medical center or nursing home facing COVID-19 with a limited supply of PPE — for instance 1,000 N95s — and having a solid PPE conservation/recycling program in place, the same facility would now have the equivalent of 4,000 masks.
Itzkowitz further broke down what those results might mean.
“Nurse Jones can write ‘Nurse Jones’ with a Sharpie on her mask and write ‘Nurse Jones’ on a brown paper bag, bring it to a dry heat sterilizer and run that mask time and time again at a 100°C for 30 minutes and keep reusing a disinfected mask,” he said. “But it’s got to be a 3M 1860 or 1870 mask because that’s what we tested.”
Limited to 3M masks
As of now, tests have only been conducted on 3M N95 masks, so Davis said he is unsure if the results could be extrapolated to other brands, so more tests will need to be conducted.
But with the voracious need for PPE, the use of the Gruenberg products for sterilization is a path TPS is diligently pursuing in order to get FDA approval as quickly as possible, according to Kent.
“We’re looking for additional institutions and hospitals to do testing to gather more information and to help collect the validating data that can be presented to the FDA,” he said. “If we can standardize on a couple different sizes based on what we feel that the market might want to accept, then it can move through quicker.”

The Gruenberg sterilizer: Two configurations
Currently, there are two configurations for the Gruenberg sterilizer: A small tabletop model with a capacity starting at 1.25 cubic feet and a large truck-in model offering 1,000 cubic feet of sterilization capacity. The larger truck-in unit is estimated to have the capacity to sterilize 15,000 to 18,000 masks a day based on a cycle time of about an hour, according to Kent.
Gruenberg’s smaller dry heat sterilizers feature a design where the process chamber is sealed throughout the entire cycle, containing any airborne particulates and sterilizing them. Larger chamber systems are easily customized, feature intake and exhaust HEPA filters, and offer several door seal options for additional safety measures.
There is HEPA filtration on the intake and the exhaust to ensure that anything that would become airborne in the oven during the process stays there, according to Davis.
“That’s also there so we’re not introducing any pathogens by drawing the air in,” he said. “Typically, the air is drawn in from some type of an interstitial space. So, we want to make sure the air we’re bringing in is clean.”
The sterilizers also use a controller that’s easy to program and run a cycle, according to Davis.
“The operating costs of a dry heat sterilizer are much lower than a steam sterilizer,” he said.
Dry heat vs. VHP
But since it’s been proven that steam sterilization is ineffective for PPE, Davis suggested it be compared to another method that is available: the use of VHP — vaporized hydrogen peroxide.
“A company called Battelle in Ohio just commercialized and got the FDA to approve a process where they put masks in a shipping container, that door is sealed — it’s a custom door that they put on it — and they inject vaporized hydrogen peroxide into the shipping container with all these masks hanging in there,” he said. “They circulate it for a period of time, and then they have to exhaust it to recover the vaporized hydrogen peroxide, because vaporized hydrogen peroxide is extremely hazardous and deadly to people in any kind of concentration form.”
The cycle time is long, and the process is acutely hazardous, according to Davis. And the hydrogen peroxide is expensive.
“And obviously these machines are fairly large,” he said. “It’s a 20-foot shipping container that they use.”
Potential VHP problems
Another issue that may come up with the VHP technology is that it is not clear if it provides full-thickness disinfection of the N95 or only sterilizes the surface of the PPE, according to Itzkowitz.
“One of the concerns was if a mask is makeup or cosmetic-product contaminated and you are going to recycle that mask, the makeup might block vaporized hydrogen peroxide and protect viral particles from being exposed to the VHP molecules,” he said. “But we know in thermal destruction, that’s not an issue because the whole mask is heated up. So, we took masks, we painted the inside of them, especially around the edges, with lipstick and with moisturizers and with all kinds of makeup. We did another quantitative fit test on four of them after we soaked them at a 100°C for 30 minutes, and they all passed. I’m a big believer in VHP. It’s an amazing technology for cleaning stuff that’s living on the surface. If you want a 6-Log kill of something biological on a surface of something else, expose it to VHP for a prescribed amount of time. If it’s living, it won’t be after the VHP chews on it. VHP is great on stuff that’s non-autoclaveable or cannot be put into a sterilizer of any sort because it’s too big or that process is destructive to that surface.”
A 6-Log kill using VHP means that exposed biological material has been rendered 99.9999 percent dead.
The perfect situation that Itzkowitz said he would like to see is where both VHP and dry-heat sterilization could be used in tandem.
“What we don’t know is if a virus particle became embedded deeper into the matrix of the mask fibers itself, could VHP attain a 6-Log kill? That is where we don’t know if the VHP could not adequately penetrate to disinfect the deepest parts of the mask,” he said. “It can’t kill what it can’t touch. Think about the makeup in this example as well.”
But thermal destruction is a different story, according to Itzkowitz.
“In an ideal world, if a facility had the ability to both surface decontaminate with VHP and take those same masks and put them into a dry heat sterilizer, you’re getting a 6-Log kill by chemical disinfection through the VHP and a 6-Log kill through heat destruction,” he said.

Gruenberg advantages
Installation of a Gruenberg sterilizer is much less complicated than other methods because mainly all that’s needed is a power source, according to Kent.
“Our process requires electricity and an exhaust,” he said. “We also have developed a different system that is a sealed-chamber system, which just requires electricity. These are smaller systems that are very efficient to run with a very fast cycle and typically would be deployed at point-of-use rather than in a parking lot or in the back of a hospital.”
Itzkowitz believes the dry heat sterilization process could become a valuable tool in smaller sub-acute care settings where PPE is going to become an issue and where there are going to be a lot of sick people discharged from hospitals to nursing homes.
“How do you protect the staff in those hospitals with probably a much more limited supply chain of PPE than we have here at a big academic medical center?” he said. “But at low temperatures in brown paper bags, you could put this stuff in an oven at 212 degrees (F) for 30 minutes and be destructive to Coronavirus, we believe. But again, COVID-19 is still a wild card.”
Deployment timeline
Once TPS gains FDA approval of the Gruenberg sterilizer to be implemented for PPE re-use, the smaller products could be deployed immediately, according to Davis.
“The larger products would be a built-to-order scenario, and we could be making deliveries from probably as soon as 16 weeks after receipt of order,” he said.
However, there is hope that could be fast tracked if needed, according to Kent.
“We’ve been talking about trying to reduce that to be a little bit more aggressive, especially if we get a little bit more information,” he said. “We will have to work with some of the key players in the market to see whether or not we can whittle it down to a small handful of standard sizes compared to very often these larger ones that are engineered to order. A lot of the customization is because the sterilizers are being put into very small areas that have existing infrastructure in place.”
But Kent said standardization challenges will be met as work on the sterilization method progresses.
“I think one of the keys that we’ve seen in the past for Gruenberg, as well as some of the other brands that we have under the Thermal Product Solutions umbrella, is that standardization has a lot of benefits, including lowering costs and decreasing lead times and improving quality as well,” he said.
Davis said there are methods in place that might could expedite the equipment to areas hardest hit and in the greatest need.
“We have equipment available — meaning truck-in dry heat sterilizers available for deployment right away,” he said. “We also have the smaller, sealed system dry heat sterilizers available for deployment right away as well. And TPS and our company have people that would be able to support getting those installed and up and running, while also working with those parties to make sure the processes are validated and that we’re helping make sure that those masks are safe for use when they come out.”



































