
Kitchen sinks, refrigerator or oven doors, and tableware are what many people think of when they are asked what’s made from stainless steel (SS). However, stainless steels encompass a wide range of products outside the home.
The more common stainless steels are iron-based alloys with chromium and sometimes nickel added to them, and they are primarily segregated into three grades: martensitic, ferritic, and austenitic. Ferritic and austenitic alloys are not hardenable by heat treatment; martensitic grades can be quenched and tempered just like ferrous or alloy steel. Austenitic stainless steels are nonmagnetic, but they can become magnetic when heavily carburized or have received severe cold work. Ferritic and martensitic stainless steels are generally magnetic.
Within the austenitic grades of stainless steel, heat-resistant (HR) alloys find application in more industrial environments such as heat treat furnaces, refineries, and chemical/pharmaceutical plants, to name a few. In addition, the food processing industry is a large consumer of the wrought form, meaning those that are transformed into plate, sheet, tube, and pipe.
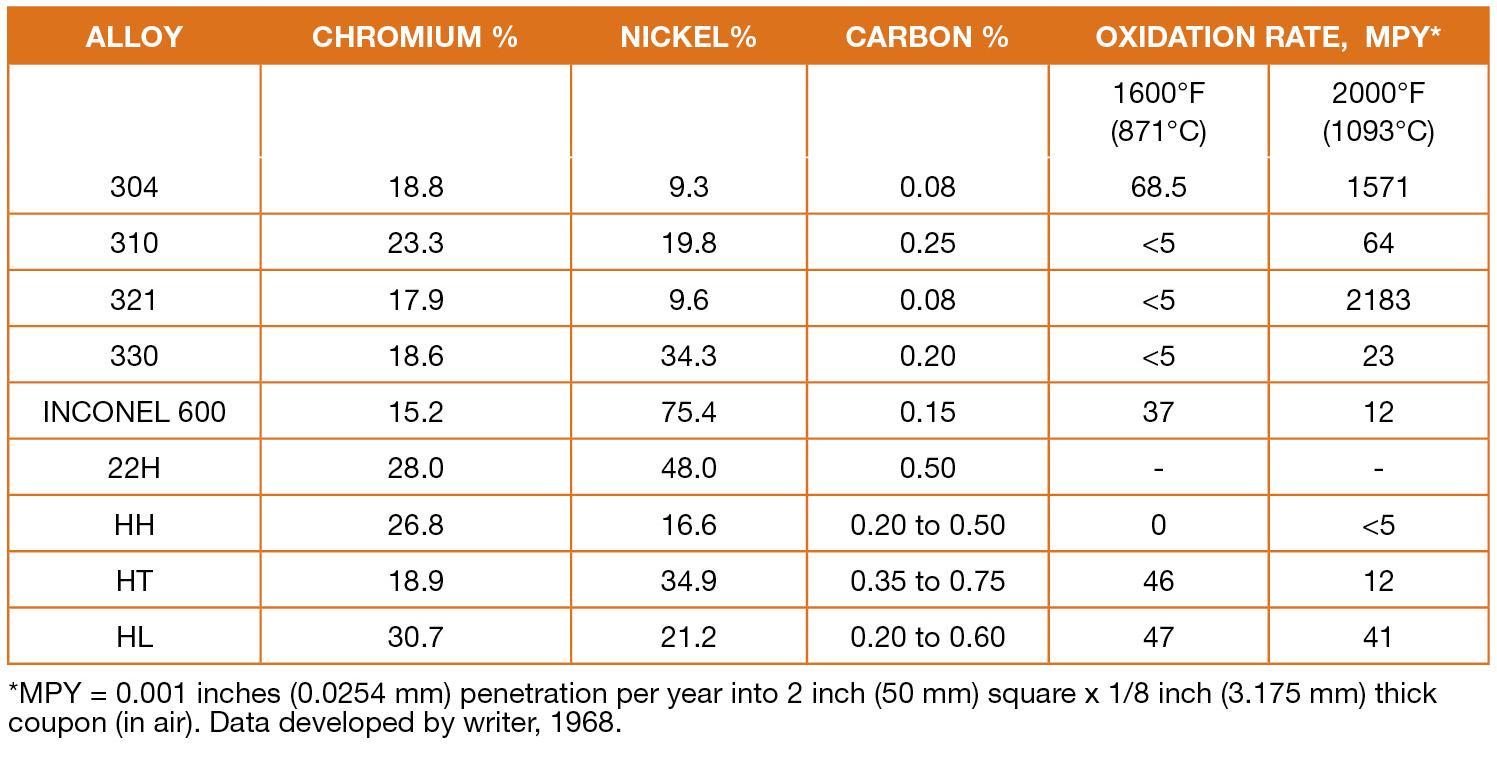
While the overwhelming constituent in steel is iron with minor additions of chromium, nickel, manganese, and molybdenum, among others, stainless steels are comprised of chromium, nickel, and the balance of iron with additions of titanium, cobalt, aluminum, and other applicant-specific elements. Stainless steels are stainless because, for all practical purposes, they don’t rust where iron and steel would. And like aluminum that receives its oxidation protection from the thin aluminum oxide layer formed in the air, stainless steels get their corrosion-resistant properties from chromium oxide. For an alloy to be considered stainless, it usually must have at least 10-percent chromium by weight. Figure 1 lists the composition and oxidation rate of some common austenitic stainless steels and heat-resistant alloys.
Martensitic stainless steel is, by its name, hardenable from the formation of martensite upon quenching, thus, in addition to the chromium, it has much higher carbon levels than the ferritic or austenitic grades. With much higher hardness and overall strength, it is used in applications where corrosion would attack alloy steel, such as in aircraft carrier-based airplane parts, tableware, kitchen knives, and surgical or dental instruments. Even iron golf club heads have been manufactured from investment cast martensitic stainless steel alloys such as 431 and 17-4 PH.
Ferritic stainless steels at room temperature have a ferritic microstructure due to their high chromium content and low carbon, thus no transformation to martensite can occur when quenched. Austenitic stainless steels have a high nickel concentration that forces an austenitic microstructure at ambient temperature. Both ferritic and austenitic stainless steels can only be strengthened by work hardening from such processing as cold forming into wire and rod — thin sheets, for example — but only to a limited degree.
Designations of wrought (sheet, plate, bar, pipe, and tube) stainless steel include: austenitic: 201, 202, and the more familiar 300 series, 302, 304, 309, 310, 312, 316, 321, 327, 330, and 347; ferritic: 405, 430, 442, and 446; and martensitic: 410, 414, 416, 420, 431, 440 A, B, and C.
440C, which is one of the more common aforementioned martensitic stainless steel grades, has a carbon level of 0.9 to 1.2 percent that’s capable of creating alloy-steel-like hardness. Its maximum corrosion resistance — like all stainless steel regardless of grade — is achieved by rapid cooling from the austenitic temperature. When austenitized at 1850°F to 1950°F (1010°C to 1065°C) and quenched and tempered, trapping the alloy in solid solution 440C produces a hardness of HRC 60.
To produce the maximum corrosion resistance, stainless steel of any grade must be rapidly cooled to prevent the premature precipitation of mainly chromium carbide plus other complex precipitates. And in specific alloys — such as a fourth group of stainless steels called precipitation hardening alloys: 17-4, 17-7, and 15-7 PH — the complex precipitates after solution and rapid cooling contribute to the strengthening and hardening of the material. The low-carbon martensite formed is the result of aging to form a precipitate of mainly copper, and the hardness is more representative of bainite (20 to 48 HRC) due in part to a very low carbon level, typically 0.07 percent.
For the industrial sector as in heat treating, the two main areas for stainless steel application are furnace interiors for structural and heating element systems. Material handling is a major consumer of heat-resistant alloys for trays and fixturing. Where wrought alloys lend themselves to easier fabrication, cast alloys are the preferred material for elevated temperatures.
Cast austenitic stainless steels employed in the heat treating industry must possess four major qualities: high-temperature strength, creep resistance, carburizing, and oxidation resistance. Interior furnace cast heat-resistant alloys can sustain continuous high-temperature exposure and generally need only sufficient creep strength. Creep is the phenomenon that causes a beam, for example, to sag (over an extended time) between two support points with an applied load much lower than would normally cause it to bend. Take alloy HK as an example, which is the cast equivalent of 310 SS. Its limiting creep stress at 1800°F (982°C) for a creep rate of 0.0001 percent is 2,500 psi — compared to its 0.2 percent offset yield strength at 1800°F (982°C), which is 8,700 psi. For design purposes, we use 50 percent of the allowable creep stress, resulting in 1,250 psi as the design limit.
Cast heat-resistant alloys used in heat treating furnaces are classified via a two-letter designation beginning with the letter H, and most have a wrought equivalent. In addition, there are dozens of wrought and cast heat-resistant alloys with a myriad of designations used in aerospace and jet engine components.
Some of the more common cast stainless steels and their wrought equivalents are: HC, 446; HD, 327; HE, 312; HF, 302B; HH, 309; HK, 310; Hl, HN, HP, HT, 330; and 22H. Figure 2 lists important thermal properties of furnace materials.
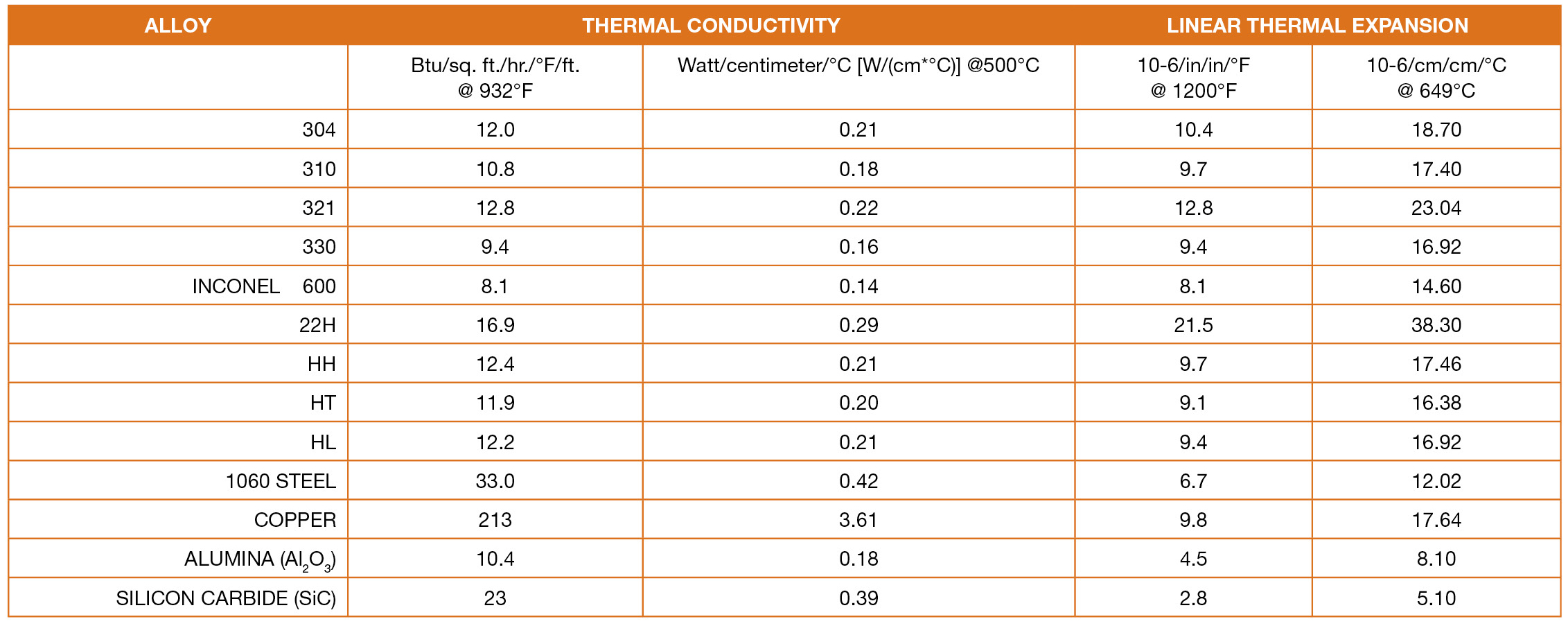
Because they are cast, the H-series alloys have higher silicon and carbon content than wrought material to assist in facilitating fluid flow during casting. The major reason for choosing cast stainless steels in furnace applications is high-temperature strength — they are intrinsically more brittle, thus they have a higher hardness than wrought material due to excess carbide created by the higher base carbon.
Another advantage of cast stainless steel is a thicker chromium oxide layer compared to wrought material that is subjected to several stages of mechanical forming that can reduce or result in thinner oxides. That oxide is the primary barrier against the carburizing process that most often reduces the life of the alloy. Carbon diffused through the oxide reacts with the chromium matrix forming chromium carbide, which reduces the chromium’s ability to form additional oxide, thereby accelerating the destruction of the component. Aluminum is often added to enhance the carburizing resistance of the alloy.
The second most detrimental influence on heat-resistant alloys is thermal cycling, and the combination of carburizing and thermal cycling can destroy alloy life. Thermal cycling leads to thermal fatigue and fatigue cracks and ultimately the breakdown of the protective oxide. If potential thermal cycling can’t be avoided, designers must take steps to reduce direct exposure to the heat source or reduce a component’s cross-section, which will reduce the temperature difference through the material attenuating the thermal stress.

An example of thermal cycling’s negative effect on heat-resistant alloys occurred several years ago on a pair of load support roller rails made from cast 22H — a 28-percent chromium, 48-percent nickel, and 15-percent cobalt alloy. The furnace was a batch-type system with a top-cool chamber and accelerated gas cooling for solution annealing 304 SS automobile exhaust pipes (the pipe from the engine to the catalytic converter). See Figure 3. The cycle consisted of heating a 36″ w x 72″ l x 36″ h load of pipes to 1950°F (1065°C), soaking one-and-a-half hours, and quenching/cooling. Immediately thereafter, another tray was charged into the hot furnace. The atmosphere consisted of nitrogen only and no carburizing gas. The unusually rapid cycling (for no other reason we can surmise) caused the roller rails (see Figure 4), to grow permanently over a six-month time period to the unbelievable length of 6 inches into the rear wall insulating fiber. The roller rails were approximately 80 inches long initially. So the 6 inches of growth was 4.6 times the 1.3 inches we expected, but surely not in just six months. It’s normal for continuous furnace trays that are heated and quenched repeatedly to experience permanent growth, but again, not to the degree seen here. We knew that thermal cycling was a possible issue, but we were shocked at the magnitude of permanent growth. We removed the added growth only to see the rail continue to grow. The solution was to replace the 22H alloy rails with high-density alumina and, eventually, silicon carbide.


Finally, another classic example of thermal fatigue in heat-resistant alloys is shown in Figure 5. Recirculation fans are a consumable item in high-temperature furnaces, but they provide a necessary assist in uniformly heating parts from ambient to approximately 1400°F (760°C) where radiation becomes the dominant heat transfer mode. However, one can expect thermal fatigue when the fan is located just a few inches above the newly charged load.

























