
Aluminum’s many desirable properties, such as high strength-to-weight ratio, good corrosion resistance, ease of processing, and low cost, make it a very widely used material in many applications. Because of this wide use, designers are often challenged with selecting the “best choice” from numerous alloys and several heat-treating processing options available. The proper heat treatment of these alloys is necessary to achieve the specified properties and performance.
Aluminum in many forms has been used in aircraft since the early beginning. This is because aluminum alloys can be heat-treated to relatively high strengths, while maintaining low weight. It is easy to bend and machine, and cost of material is low. Because of these advantages, it is the most common material used in aerospace today. It is used in the manufacture of advanced commercial aircraft such as the Boeing 777, Airbus 380, and military aircraft such as the Boeing UCAV or the Boeing F/A-18 E/F.
When used in conjunction with aluminum alloys, the term ‘heat treating’ is generally restricted to solution heat treatment, quenching, and subsequent aging of aluminum alloys to increase strength and hardness. These usually are referred to as the ‘heat-treatable’ alloys to distinguish them from those alloys in which no significant strengthening can be achieved by heating and cooling. The latter, generally referred to as ‘non-heat-treatable’ alloys, depend primarily on cold work to increase strength.
In this short article, we will describe the various alloying elements that form the different aluminum alloys and classify them according to types of aluminum alloys.
Alloying Elements
Generally, cast and wrought aluminum alloys are classified either as heat-treatable (precipitation hardenable) alloys or as solid-solution alloys, as summarized in Figure 1. Wrought and casting alloys also are further designated by temper codes to indicate condition and the prevalent strengthening mechanism briefly as follows:
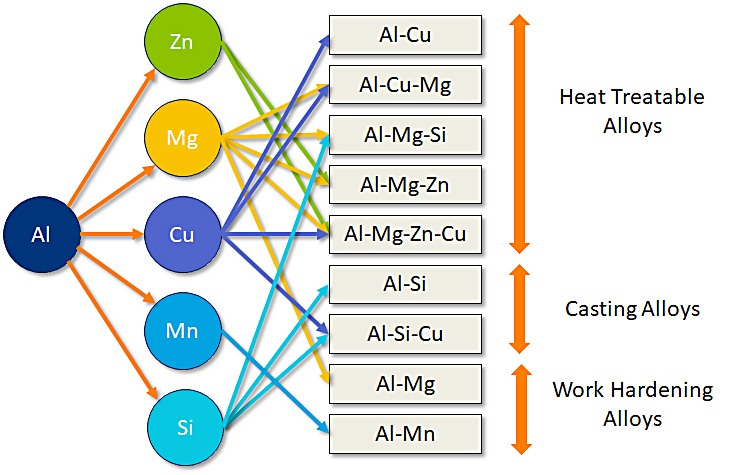
Non-age-hardening alloys are solid solution strengthened and are indicated by an as-fabricated temper (F) or annealed temper (O).
Wrought solid-solution alloys may be further strengthened by work-hardening (H-tempers).
Age-hardening alloys are precipitation strengthened indicated by heat-treatment tempers (T-tempers).
Depending on the alloying elements, strengthening of aluminum can be achieved by heat treatment or by solid solution strengthening (often in conjunction with work hardening). The elements most commonly present in commercial aluminum alloys for strengthening are copper, magnesium, manganese, silicon, and zinc. Minor alloying elements, which are added for special properties or metallurgical effects, include: Fe, Li, Ti, B, Zr, Cr, V, Sc, Ni, Sn, and Bi. In addition, silicon, which has a eutectic reaction (at 577°C with 12.6 wt% Si) and is added to improve the fluidity during casting and is thus an alloying constituent in casting alloys.
Elements and combinations that form predominantly second-phase constituents with relatively low solid solubility include iron, nickel, titanium, manganese, and chromium, and combinations thereof. Manganese and chromium are included in the group of elements that form predominantly second phase constituents.
Copper is one of the most important alloying ingredients for aluminum, because of its appreciable solubility and its strengthening effect from precipitation hardening. The binary Al-Cu system is the classic example of precipitation hardening, and the age hardening of binary aluminum-copper alloys is one of the most studied systems.
Most commercial Al-Cu alloys contain other alloying elements. Aluminum-copper alloys containing 2 to 10 percent Cu, generally with other additions, form important families of alloys. The aluminum-copper system is the basis for the wrought 2xxx and cast 2xx.x alloys, and many other heat-treatable alloys contain copper. Most contain other alloying elements such as magnesium.
The main benefit to adding magnesium to aluminum-copper alloys is the increased strength possible following solution heat-treatment and quenching. In both casting and wrought alloys, as little as 0.05 percent magnesium is effective in changing aging characteristics.
The Al-Mg system is the basis for the wrought 5xxx and cast 5xx.x non-heat-treatable aluminum alloys. The addition of magnesium provides solid solution strengthening without unduly decreasing the ductility. The Al-Mg alloys offer an excellent combination of solid-solution strengthening, corrosion resistance, and the strengthening of wrought alloys by work hardening.
Silicon is a ubiquitous impurity in commercial aluminum alloys. Impurity levels in electrolytic commercial aluminum in the range of 0.01 to 0.15 wt% Si, and the presence of iron greatly reduces the solubility of silicon in aluminum.
As an alloying element, the outstanding effect of silicon additions to aluminum and its alloys is the improvement in casting characteristics. Aluminum-silicon alloys that do not contain copper additions are used when good castability and good corrosion resistance are needed.
Aluminum-copper-silicon alloys are the most widely used aluminum casting alloys. The amounts of both additions vary widely, so that the copper predominates in some alloys and the silicon in others. In these alloys, the copper contributes to strength, and the silicon improves castability and reduces hot shortness. Aluminum-copper-silicon alloys with more than 3 to 4 percent Cu are heat treatable, but usually heat treatment is used only with those alloys that also contain magnesium, which enhances their response to heat treatment.
The Al-Mg-Si system also is the basis for the heat-treatable 6xxx series of heat-treatable wrought alloys. The wrought Al-Mg-Si (6xxx) alloys contain up to 1.5 percent each of magnesium and silicon in the approximate ratio to form Mg2Si, that is, 1.73:1.

The aluminum-zinc alloys have been known for many years, but hot cracking of the casting alloys and the susceptibility to stress-corrosion cracking of the wrought alloys curtailed their use. Zinc confers little solid solution strengthening or work hardening to aluminum, and no significant technical benefits are obtained by the addition of just zinc to aluminum. However, the addition of copper and/or magnesium with zinc results in attractive compositions for heat treating or natural aging. Usually, other elements, such as chromium, are also added in small quantities.
The addition of magnesium to the aluminum-zinc alloys develops the strength potential of this alloy system, especially in the range of 3 to 7.5 percent Zn. Magnesium and zinc form MgZn2, which produces a far greater response to heat treatment than occurs in the binary aluminum-zinc system. The Al-Zn-Mg precipitates provide the basis for the 7xxx wrought alloys and the 7xx.x cast alloys.
The addition of copper to the Al-Zn-Mg alloys results in the highest-strength aluminum-base alloys commercially available. In this alloy system, zinc and magnesium control the aging process. The effect of copper is to increase the aging rate. Copper also increases quench sensitivity upon heat treatment. In general, copper reduces the resistance to general corrosion of Al-Zn-Mg alloys but increases the resistance to stress corrosion.
Lithium reduces the density and increases the modulus of aluminum alloys. In binary alloys it forms metastable Al3Li precipitates and combines with aluminum and copper in Al-Cu-Li alloys to form many Al-Cu-Li phases. Because of its high cost relative to other alloying elements, lithium alloys have been found to be cost effective thus far only in space and military applications. Some applications in sporting equipment such as bicycle frames or baseball bats have also been used.
Titanium (Ti) is used primarily as a grain refiner of aluminum alloy castings and ingots. The grain-refining effect is enhanced with boron. Titanium depresses the electrical conductivity of aluminum, but its level can be reduced by the addition of boron, which forms insoluble TiB2.
Zirconium (Zr) additions in the range of 0.1 to 0.3 percent are used to form a fine precipitate of intermetallic particles that inhibit recovery and recrystallization. Most 7xxx and some 6xxx and 5xxx alloys developed since the 1960s contain small amounts of zirconium, usually less than 0.15 percent, to form Al3Zr dispersoids for recrystallization control.
An increasing number of alloys, particularly in the Al-Zn-Mg family, use zirconium additions to increase the recrystallization temperature and to control the grain structure in wrought products. Zirconium additions leave this family of alloys less quench sensitive than similar chromium additions.
Zirconium additions have been used to reduce the as-cast grain size, but its effect is less than that of titanium. In addition, zirconium tends to reduce the grain refining effect of titanium plus boron additions, so that it is necessary to use more titanium and boron to grain refine zirconium-containing alloys.
Conclusion
In this short article, we have discussed the various alloying elements that are used in aluminum alloys and described why they are added. In the next articles we will dive deeper into the heat treatment of aluminum and describe some of the problems and solutions to those problems.
As always, should you have any comments regarding this article, or suggestions for other articles, please contact the editor or author.
References
- R. Davis, Aluminum and Aluminum Alloys, vol. ASM Specialty Handbook, Metals Park, OH: ASM International, 1993.

























