
Last month, we examined the history and heat-treatment of aluminum and how it applies to the quality functions of the industry. This month, we will do the same for the history and heat-treatment of steel. There are many roads of scientific achievement that have led to our current state (all of which will not be covered in this article).
A Brief History
Steel, in many forms, has a long history. From ~500bce when Egyptians austenitized steel, to sixth century BC southern India smelting “wootz” steel, to recent studies of 11th century Damascus steel containing carbon nanotubes (Figure 1), to the invention of the open-hearth furnace in 1865, steel has had a colorful history. I won’t be spending too much time on its history but noting significant events which enable steel to be produced efficiently with a high grade of quality we see today.
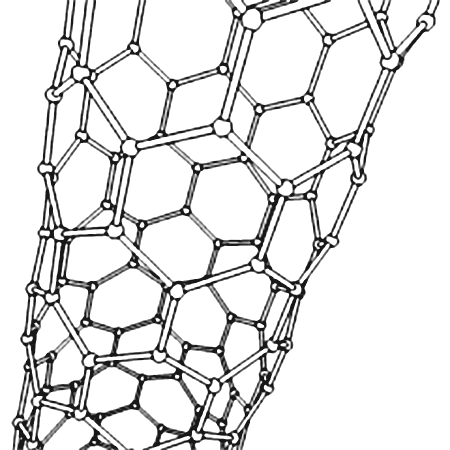
The history of steel has several lines of lineage converging to present day. Certain significant events took place which have influenced how we produce and heat-treat steel in modern times. As stated, Egyptians’ austenitization of steel did not include a carbon source. Their process was used primarily for weapons and tools. It seems the first source of carbon in steel production/heat treatment was a consequence of the first blast furnace in China around the sixth century BC. China, though, was not the only country producing steel implements and tools. In ancient India, around the sixth century BC, craftsmen in southern India used crucibles to smelt wrought iron with charcoal to produce what is called “wootz” steel. Wootz steel is a material that was admired for its sharp and tough nature. The most distinguishing feature is the swirling patterns cause by bands of clustered *Fe3C particles (Fe3C is called cementite). It is an intermetallic compound of iron and carbon (an intermediate transition metal carbide). It is 6.67% C wt% and 93.3 percent Fe wt%) (Figure 2).

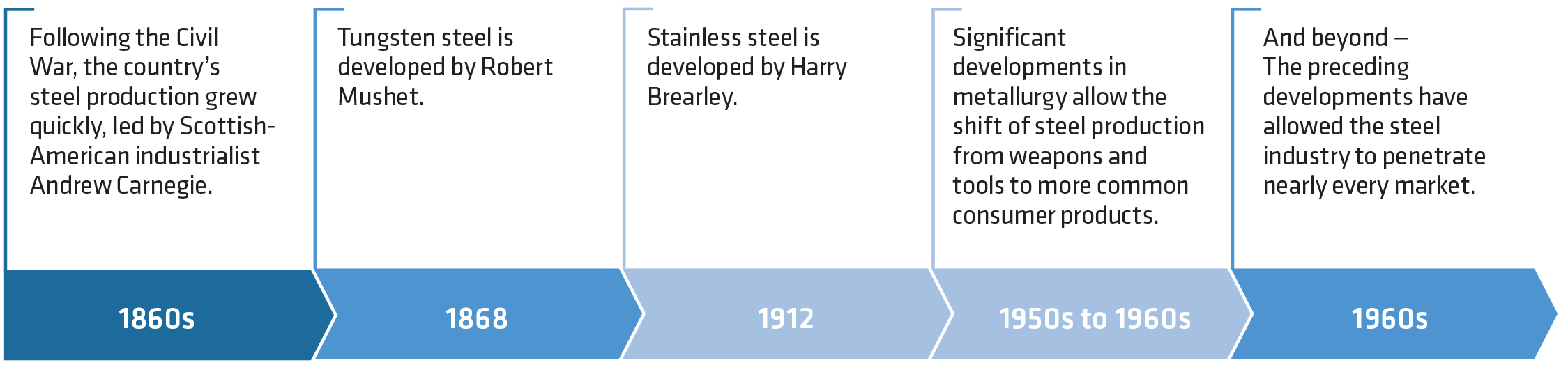
By the 12th century, Sri Lanka was the world’s largest producer of crucible steel. It supplied steel ingots to Asia as well as the Middle East. By the 18th century, steel was becoming widely renowned for its durability and extent of use. Due to limited production and less robust production processing, steel was very expensive. Its purchase was limited to the end use of special applications such as weapons, armor and tools. As the industrial revolution progressed, the production and advancement in steel technology moved quickly. In 1855, the Bessemer process was introduced. The Bessemer process’ key principle is forcing hot air through the molten metal, which in turn removes impurities. In 1865, the open-hearth furnace was invented through a joint venture between Sir Carl Wilhelm Siemens and French engineer Pierre-Emile Martin. In this process, excess carbon and other impurities are burnt out of pig iron to produce steel. The open-hearth technique overcame the insufficient temperatures generated by normal fuels and furnaces, enabling the steel to be produced in bulk for the first time. The rest is recent history (Figure 3).
The Science
Scientific principles link the processing parameters to structure and properties and are increasingly necessary for proper application of the equipment and instrumentation.
An example of scientific efforts that directly support the advancement of heat-treat technology includes the characterization of mechanisms of phase transformation which produce desired structures and properties of heat-treated steel.
Before studying the heat treatment of steel, it is helpful to explain what steel is. Fundamentally, all steels are alloys of iron, carbon, manganese, silicon, sulfur, and phosphorus. Carbon content in commercial steels usually ranges from 0.05 wt% to 1.0 wt%. The broad possibilities with regards to steel and its use are attributed to two main characteristics:
- Iron is an allotropic element. (Allotropy is the phenomenon of an element having different crystal lattices depending on the particular temperature and pressure.)
- The carbon atom is much smaller than the iron atom.
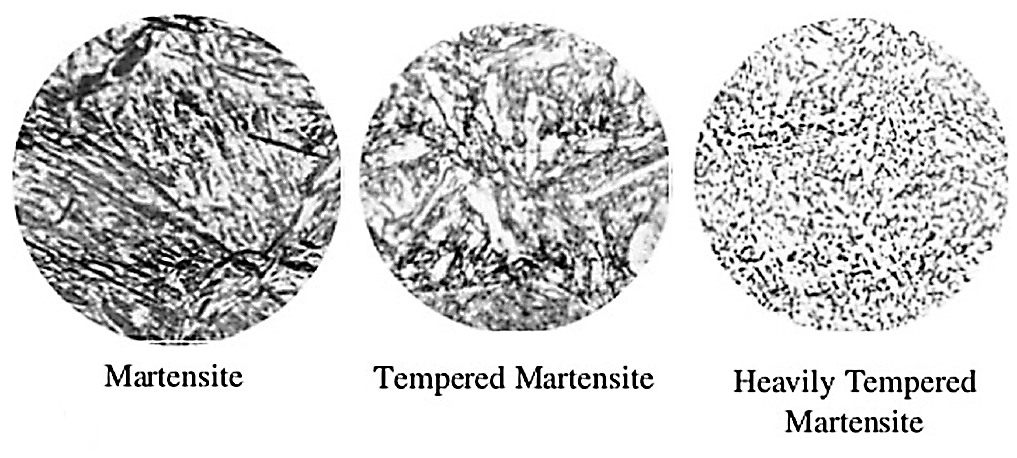
The alloying mechanism for iron and carbon is different from most other alloy systems in that the alloying of iron and carbon occurs in a two-step process. In the initial step, iron combines with 6.67 percent carbon, forming iron carbide, which is called cementite. At room temperature, conventional steels consist of a mixture of cementite and ferrite. Each of these is known as a phase (defined as a physically homogeneous and distinct portion of a material system). When a steel is heated above 1,340°F, cementite is dissolved into a new matrix phase called austenite. Austenite is designated as a solid solution of one or more elements in face-centered cubic iron (gamma γ-iron). Unless otherwise designated (such as nickel austenite), the solute is generally assumed to be carbon. The next phase of transformation, martensitic transformation, takes place when the steel is cooled rapidly. When alloys are cooled rapidly, the carbon atoms cannot make an orderly escape from the iron lattice. This results in distortion of the lattice which manifests itself in the form of hardness and/or strength. If cooling is fast enough, a new phase known as martensite forms. Eventually, the martensite decomposes into a mixture of ferrite and cementite if heated below the A1, and this structure is referred to as tempered martensite (Figure 4).
After this comes the crystal structure and phases (bcc to α-Fe) of which are not as pertinent to this article and would take quite a bit of extra time to go into.
Heat Treat Key-Process Characteristics
Ensuring the material being heat-treated is of the required composition is critical to successful and repeatable results. When material is received, there should be a verification process in place that ensures a quality representative (or delegate) verifies material composition against the purchase order.
A simple visual comparison using the purchase order and the material certification is enough to ensure the material is conforming.
Furnace pyrometry testing is key to ensuring that:
- The furnace systems are in working condition.
- Consistent conforming results will be obtained on all product processed in the furnace.
Pyrometry results should be reviewed carefully by quality to ensure conformance. Any deviations should be investigated promptly before processing further production. Subsequent pyrometry testing should be performed at the required frequency per AMS2750 to ensure consistent results.
Summary
For those of us involved in quality as it applies to heat treat, it is essential to have a good understanding of the process. This knowledge will help the quality team in the disposition and process approval.


























