
The major earthquake and tsunami that occurred in Japan in 2011 did serious damage to boiling water reactors of the Fukushima-Daiichi nuclear power plant. The accident was a loss-of-coolant accident event. The accident forced the international nuclear community to search for a new fuel configuration that would be more resistant to a loss of coolant accident than existing zirconium/UO2 pellets [1]. The new strategy is to develop a fuel system that can tolerate and perform well in severe accident scenarios not comprising the reactor performance [2].
To develop an accident-tolerant fuel (ATF) system, there can be three possible methods. The first is to develop the existing Zr alloys to enhance stability and performance in high temperatures [3]. The second way is to choose a different cladding other than zirconium alloys with higher performance and oxidation resistance [2]. The third is to identify a fuel form consisting of additional barriers that can withhold the fission products [4].
The possible accident tolerant fuel material must have: (i) enhanced kinetics with steam, (ii) slower hydrogen production rate, (iii) improved retention of fission products, and (iv) improved cladding and fuel preposition [5]. In the event of loss of active cooling, if a cladding material can tolerate the reactor core for considerably longer time while maintaining the reactor performance then it can be considered as ATF cladding material [5]. Some of the leading materials to replace zirconium as cladding materials are alumina forming ferritic alloys and silicon composites [6].

Kanthal® APMT is an advanced powder metallurgical FeCrAlMo-based alloy developed by Sandvik material technology group. Chemical composition of the alloy determined via optical emission spectroscopy is listed in Table 1. The alloy contains dispersoids and constituent elements that form stable high temperature alumina and provide higher mechanical strength compared to traditional FeNiCr/NiCr alloys. Formation of dense and highly adherent alumina on the surface provides better oxidation protection than chromia layer. Apart from its traditional usage in various forms, such as high temperature crude processing tubes, heating elements, heat exchangers, etc., APMT is considered for ATF cladding material in nuclear power plants. Zirconium-based alloys are widely used as nuclear fuel cladding material; however, the alloys have higher reaction kinetics with steam and form hydrogen at elevated temperature — a major concern during a loss of coolant accident. Although Kanthal APMT has nearly 10 times the neutron absorption cross section of Zircaloy-2: 2.47 vs. 0.20 barns, it has good corrosion resistance and higher temperature stability property to act as a cladding material. This explains the versatility shown by Kanthal APMT at higher temperatures and extreme conditions. Advancement in fuel enrichment and fuel rod geometries can minimize the effect of higher neutron absorption.
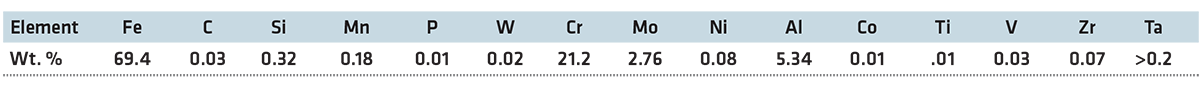
Oxidation property
Kanthal APMT has superior oxidation resistance at high temperatures. A study conducted by Sandvik [7] at 1,100°C (2,012°F) showed formation of an adherent passive alumina barrier layer and no mass gain for exposure of up to 900 hours, while high performance Fe35Ni25Cr alloys had corroded on the surface, see Figure 1, and had significant mass gain. Formation of a chromia and alumina oxide layer depends under different environment conditions. Figure 2 shows the oxide development on APMT under normal reactor conditions and accident conditions [1].

The oxide layer that gets developed under 288/300°C (550/572°F) water is a chromia layer. If the same experiment conditions have excess hydrogen, then a pure chromia oxide gets developed in the surface whereas, in excess of oxygen, Rebak [1] found that there was formation of dual oxide. The inner oxide layer was a chromia layer whereas the external layer consists of chromium and iron with no aluminum or molybdenum. When exposed to a steam-oxidizing environment at 1,200°C (2,192°F), a pure alumina layer develops on the surface. On being air-oxidized rather than steam-oxidized at 1,200°C (2,192°F), APMT grows a thicker and wider oxide layer than the steam-oxidized APMT [8, 9]. There is faster oxide growth on air-oxidized APMT than steam-oxidized APMT when exposed to respective corrosion environment.
Both the oxide layer consists of columnar grains. The higher frequency of grain boundary diffusion in APMT causes the rapid oxidation rate in air for APMT [10]. The evolution of oxides on the surface of APMT depends on the changing environment where it is exposed. Rebak [1] tested the alloy from water to steam and steam to water to test the stability of a pre-oxidized layer on the surface. It was found that the chromia layer on a pre-oxidized APMT was replaced by a pure alumina layer when exposed to high temperature steam whereas the alumina layer on a pre-oxidized APMT was replaced by a pure chromia layer when exposed to high temperature water. This explains the versatility of oxides formed by APMT in different corrosion scenarios and shows the benefits of using APMT as cladding material in the nuclear reactors. This key property shown by APMT would be very vital in dealing with loss-of-coolant accident situations as the material can switch the formation of oxide layer according to its environment.
Creep property
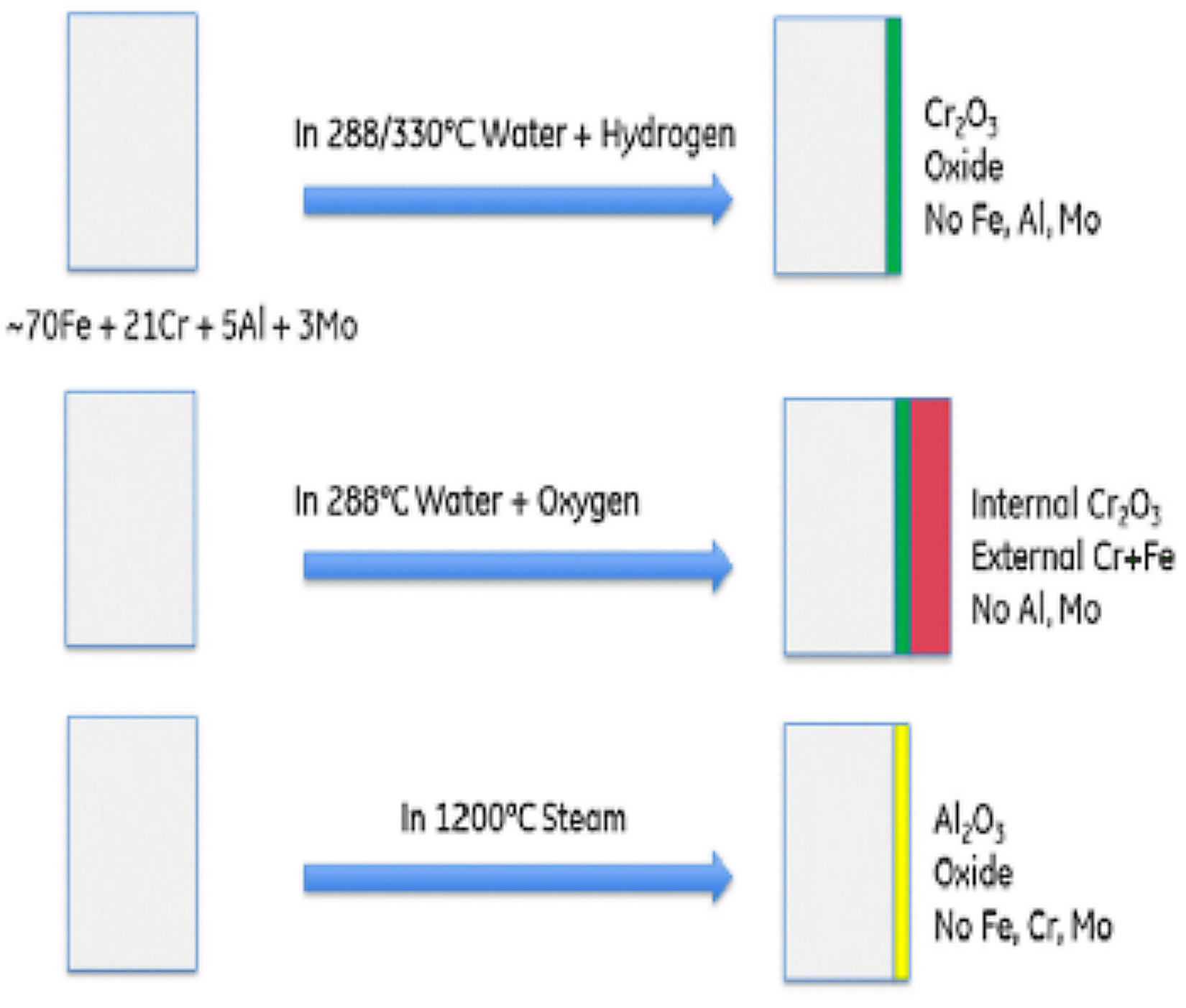
Creep deformation and rupture of metals is of great concern for prolonged service life at elevated temperatures. For an engineering structure operating in ambient temperature, creep is not considered as a significant deformation mechanism. However, in high-temperature applications, parameters like the duration of test, the grain size, subgrain size, precipitate size, effective stress, and environment all have a role in dictating the strength of the material. Creep is time-dependent plastic deformation in materials under constant load or stress, specifically observed at higher homologous temperatures. Kanthal APMT is likely to reach creep regime temperatures even under normal operating conditions.
A typical example of a creep curve for a Kanthal APMT specimen tested at 973 K (1,292°F) and 100 MPa is shown in Figure 3a, and the corresponding variation of creep rate versus time plotted in double logarithmic scale is shown in Figure 3b. The sample reached steady state creep rate in the 10-6/s range. The creep curve consisted of three distinct regimes: primary, secondary (steady state), and tertiary. During the primary creep, the creep rate decreases with increasing strain due to work hardening via dislocation multiplication and interactions. In the secondary stage, creep rate is stabilized as the work hardening effect is counter balanced by the dislocation annihilation and rearrangement.
The secondary stage, also known as steady-state creep, is of vital importance as it sheds light on the creep deformation mechanism. In the secondary creep stage, the material deforms plastically while there is a higher degree of strain hardening and increased dislocation density, resulting in a constant creep rate. Strain rate increases with time in tertiary creep. Increased strain rate results in necking, crack, and void formation. Finally, the creep rate accelerates as cavities start growing, leading to the tertiary stage and rupture ultimately. Tertiary creep stage is rapid, so engineering structural materials are designed not to enter this stage.
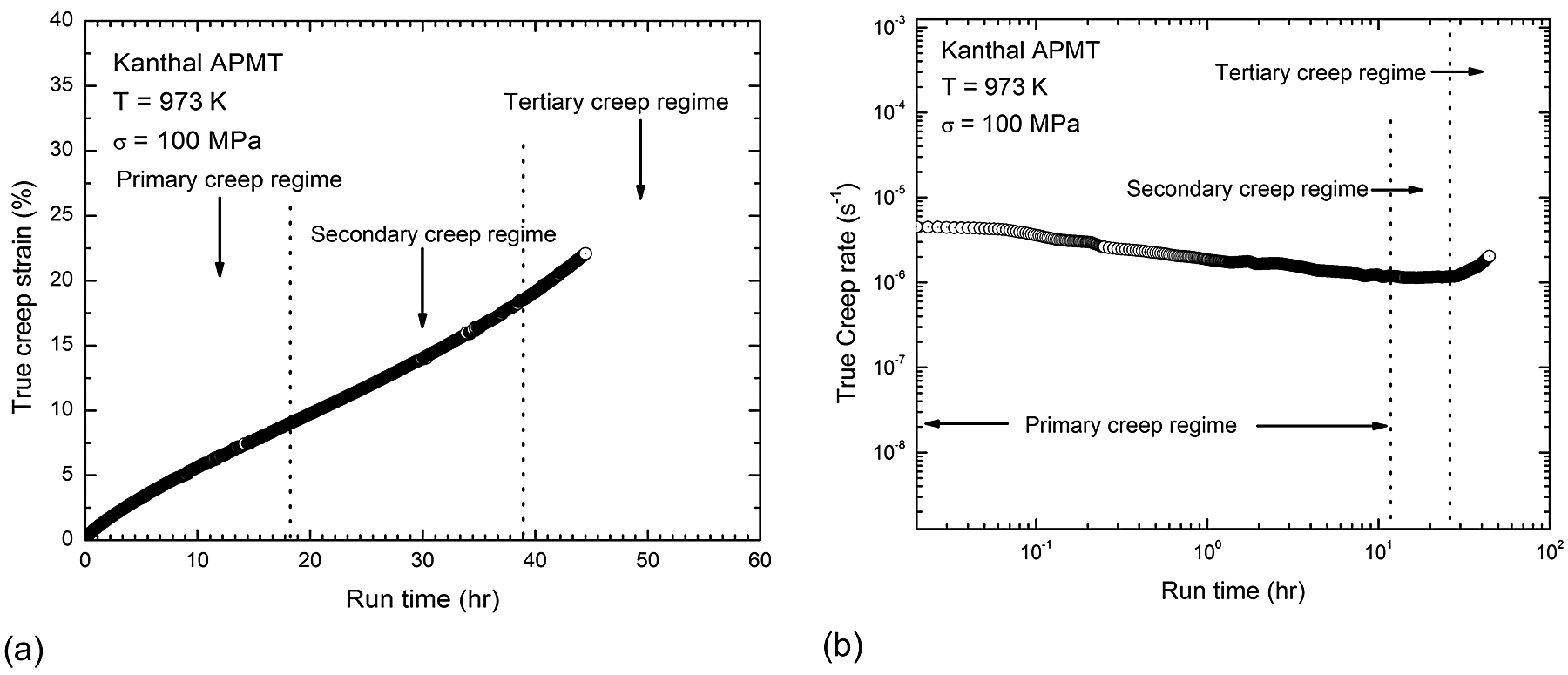
The solid solution strengthening, fine substructure, dense dislocation network, and dispersoids increase the creep resistance of Kanthal APMT. These strengthening mechanisms deteriorate over time during creep, and the material loses its inherent creep rupture strength. The creep strength of an alloy depends on its ability to withstand temperature and stress for a prolonged period. The formation of dense adherent chromia and alumina passive barrier protect the material from high temperatures degradation. Microstructure of a sample crept at 125 MPa and 923 K (1,202°F) was examined via optical microscopy. Figure 4 shows optical micrographs at various locations along loading direction of a sample crept at 125 MPa and 923 K (1,202°F). At the tip of the fracture surface, extensive microstructural damage was observed with porosities, cavities, carburization, and no defined grain structure. At quarter of fracture length away from the fracture surface, elongated grains with high aspect ratio along axial direction, increased carburization, and porosity were observed. At halfway from fracture surface, elongated grains along axial direction, increased carburization, and porosity were observed. Toward the grips equiaxed microstructure like as-received condition was observed.
Conclusion
Kanthal APMT is candidate material for accident-tolerant fuel cladding in nuclear energy application. Traditional Zr-based alloys used for fuel cladding, though they have lower neutron cross section, have lower thermal stability at extremely high temperatures, especially in case of loss-of-coolant accident. The alloy has excellent high temperature oxidation resistance as it can form dense, adherent, passive chromia and alumina layers depending on its environment. Creep study of the alloy showed reasonable high temperature/high stress creep property. The alloy needs to be further studied in irradiated and unirradiated conditions prior to industrial application.
Reference
- R. B. Rebak, “Versatile oxide films protect FeCrAl alloys under normal operation and accident conditions in light water power reactors,” vol. 70, no. 2, pp. 176–185.
- A. Naceur and G. Marleau, “Neutronic analysis for accident tolerant cladding candidates in CANDU-6 reactors,” vol. 113, pp. 147–161.
- S. J. Zinkle, K. A. Terrani, J. C. Gehin, L. J. Ott, and L. L. Snead, “Accident tolerant fuels for LWRs: A perspective,” vol. 448, no. 1, pp. 374–379.
- K. A. Terrani, L. L. Snead, and J. C. Gehin, “Microencapsulated fuel technology for commercial light water and advanced reactor application,” vol. 427, no. 1, pp. 209–224.
- R. B. Rebak, “Alloy selection for accident tolerant fuel cladding in commercial light water reactors,” vol. 2, no. 4, pp. 197–207.
- K. A. Terrani, “Accident tolerant fuel cladding development: Promise, status, and challenges,” vol. 501, pp. 13–30.
- 6-C-1-3 APMT tube datasheet, www.kanthal.com
- T. M. Copeland-Johnson, C. K. Nyamekye, S. K. Gill, L. Ecker, N. Bowler, E. A. Smith, and R. B. Rebak, “Characterization of kanthal APMT and t91 oxidation at beyond design-basis accident temperatures,” vol. 171, p. 108598.
- “Oxidation characteristics of two FeCrAl alloys in air and steam from 800°c to 1300°c | SpringerLink.”
- Hauffe, “High temperature oxidation of metals,” P. Kofstad John Wiley and Son, New York 1966, 340 s,” vol. 18, no. 10, pp. 956–957. eprint: https://onlinelibrary.wiley.com/doi/pdf/10.1002/maco.19670181017.
























