
In my article last month, I discussed the alloying elements used in aluminum alloys. In this article, I will be discussing the solution heat treatment of aluminum. In subsequent articles, I’ll discuss the other unit processes of aluminum heat treating.
Heat Treating Aluminum Alloys
Aluminum alloys are classified as either heat-treatable or not heat-treatable, depending on whether the alloy responds to precipitation hardening. In the heat-treatable alloy systems such as 7XXX, 6XXX, and 2XXX, the alloying elements show greater solubility at elevated temperatures than at room temperature. The general sequence of heat-treating aluminum is shown in Figure 1. The temper designation describes fully the process sequence. The temper designations will be discussed in a later article on aging.
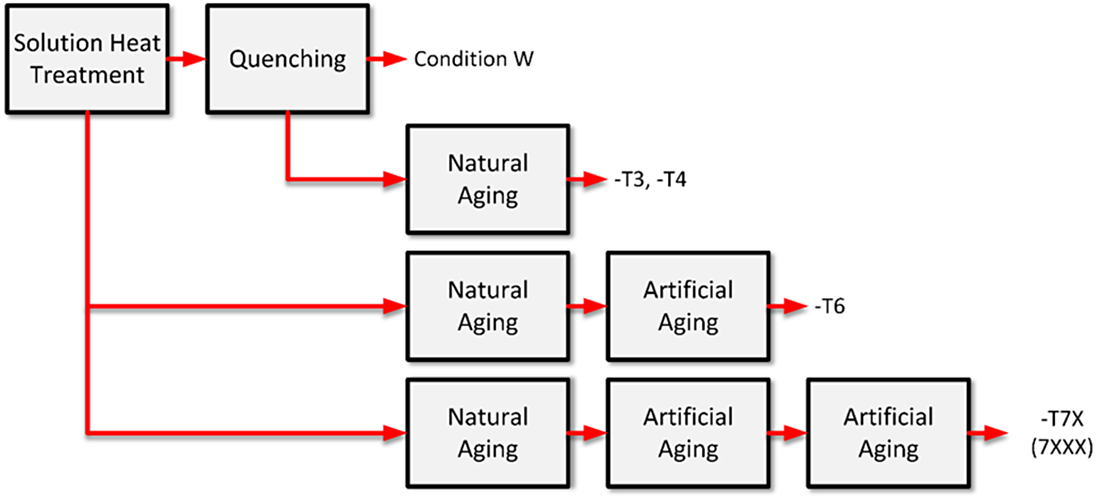
Solution Heat Treatment
The purpose of the solution heat treatment is to obtain the maximum practical solid solution concentration of the hardening solutes such as copper, magnesium, silicon, or zinc. The solubilities of these elements increase markedly with temperature, especially just below the eutectic melting temperature. Consequently, the most favorable temperature for solution treatment is close to the eutectic temperature, typically only 5° to 8°C (10° to 15°F) below the eutectic temperature.
Solution treating is done close to the eutectic temperature (Table 1), therefore good control, and uniformity of temperature within furnaces are essential to avoid incipient (eutectic) melting in grain boundary regions. This can result in a decrease in both strength and ductility. In addition, quench cracking is sometimes encountered when the normal solution temperature is exceeded.
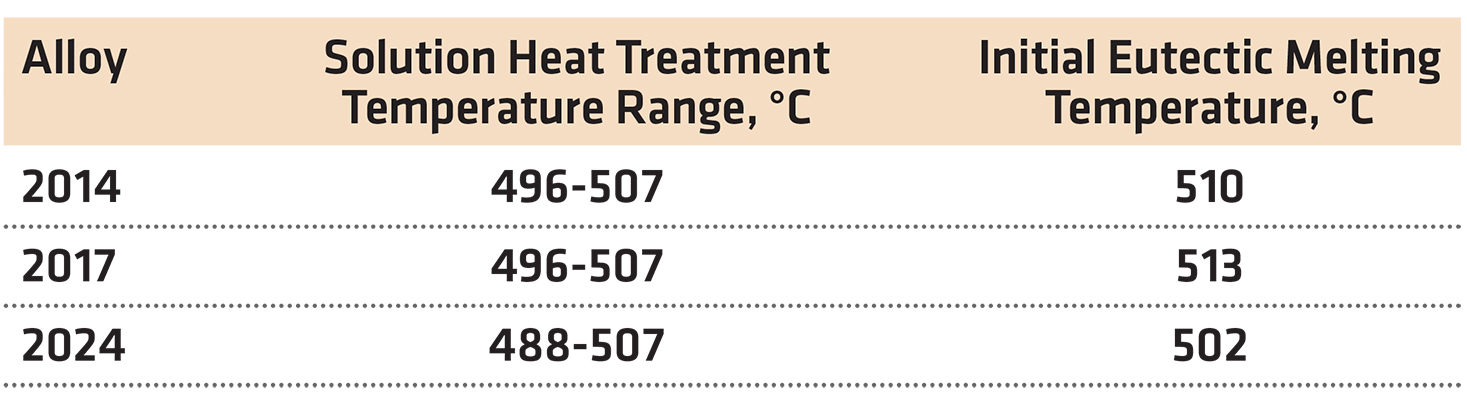
Tight temperature control is particularly critical for 2xxx alloys such as 2014, 2017, and 2024, for which the initial eutectic melting temperature is only a few degrees higher than the recommended maximum solution temperature. More latitude in the solution heat-treating temperature is generally permissible with the 7xxx series than with the 2xxx series alloys.
Increasing the solution heat-treating temperature from 350°C up to the limit of solubility, then quenching and artificially aging, tends to increase the mechanical properties of the alloy. After the solubility limit is reached, there is little benefit to increased solution heat-treating temperatures [1] [2] [3]. However, increasing the solution heat-treating temperature has been reported to accelerate aging [4], and provide an increase of hardness [5].
The time required at the solution heat-treating temperature depends upon type of product, alloy, casting, or fabricating procedure used, and section thickness. Typical soak times are shown in Figure 2. Air is the usual heating medium, but molten salt baths or fluidized beds are advantageous in providing more rapid heating. High-temperature oxidation, evidenced by the formation of small rounded voids or crevices within the metal and by surface blisters, may occur because of heating aluminum products at solution heat treating temperatures in moisture-laden atmospheres.
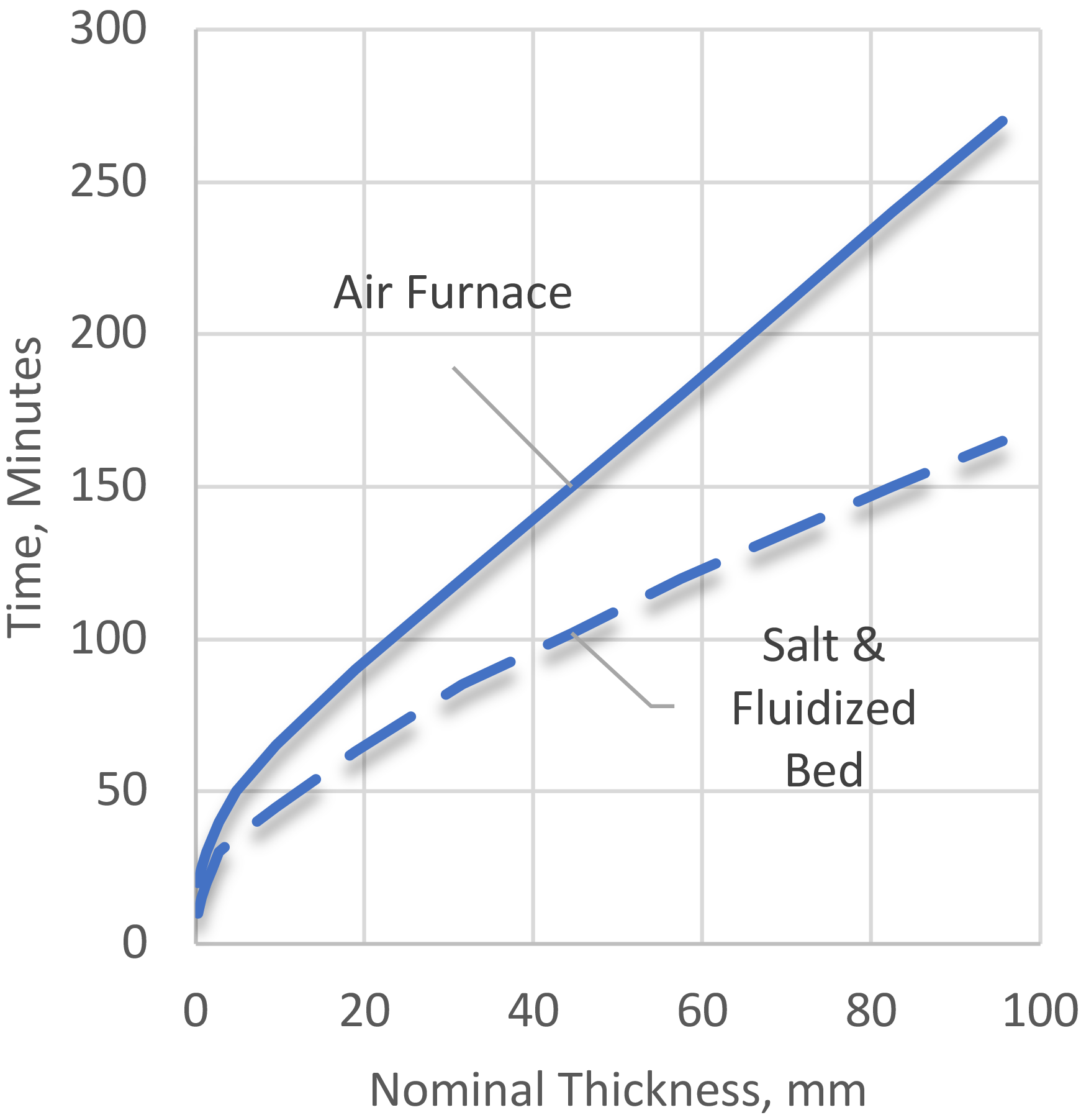
The effect of time at the solution heat-treating temperature on increasing mechanical properties has been studied extensively [1] [6]. There is little benefit to extended solution heat-treating times. The long times generally cited in product heat-treating specifications are to ensure that the entire product with a given heat-treating load reaches the process temperature. Increased time can cause increased oxidation, blisters, and a decrease in properties from grain growth [7]. Excessive soak times can also lead to excessive diffusion of the aluminum-clad surface [8]. Reheat treatment of clad products thinner than 0.75 mm (0.030 in.) is generally prohibited by specifications.
Heating rate is often cited for causing incipient melting [9]. This is related to the S Phase (Al2CuMg). The S phase is slow to dissolve. This can result in local non-equilibrium melting at temperatures from 475° to 490°C if the product is heated rapidly [10] [11] [12]. At slow heating rates, the S phase (Al2CuMg) has time to dissolve in the matrix, and incipient melting is not observed. Incipient melting in an aluminum alloy is shown in Figure 3.
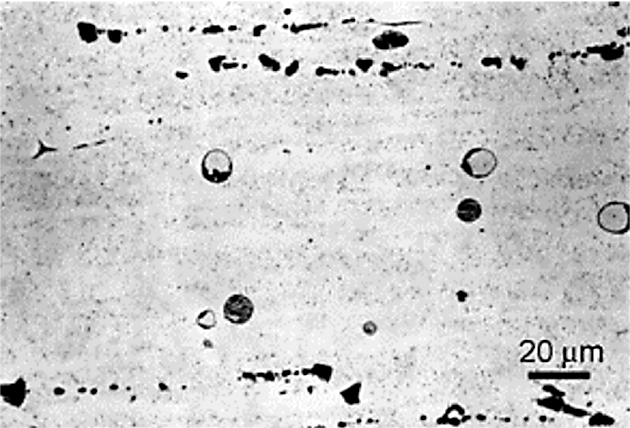
Eutectic melting is often not apparent until tensile testing or metallographic examination is performed. This is usually manifested by low elongation or poor ductility. Luckily, eutectic melting is often accompanied by high-temperature oxidation.
High-temperature oxidation is a misnamed condition of hydrogen diffusion that affects aluminum surfaces at elevated-temperature [13]. When moisture encounters aluminum at high temperatures, atomic hydrogen is formed. This hydrogen can diffuse into the metal and collect at grain boundaries and lattice defects. This results in surface blistering and porosity (Figure 4 and Figure 5).

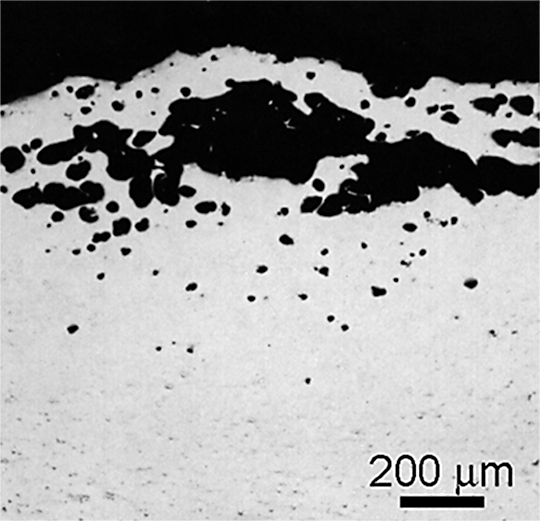
Not all alloys and product forms are equally vulnerable to this type of attack. The 7xxx series alloys are most susceptible, followed by the 2xxx alloys. Extrusions are the most susceptible form; forgings are second. Low-strength alloys and alclad sheet and plate are relatively immune to high-temperature oxidation.
Moisture can be minimized by thoroughly drying parts and racks before they are charged. Drain holes often are needed in racks of tubular construction to avoid entrapment of water. Another common requirement is adjustment of the position of the quench tank with respect to furnace doors and air intake to reduce moisture entrainment by the furnace.
The most common method to alleviate blistering is use of a protective compound such as ammonium fluoroborate in the furnace [14]. Such a compound usually is effective in minimizing the harmful effects of moisture and other undesirable contaminants because it forms a barrier layer or film on the aluminum surface. Other methods include anodizing the aluminum surfaces.
Conclusions
In this short article we have described the basic principles of the solution heat treatment of aluminum. Several problem areas have been identified, and possible corrective actions have been detailed.
Should there be any comments on this article, or suggestions for other articles, please contact the editor or the author.
References
- P. Brenner, Luftwissen, vol. 7, p. 316, 1940.
- B. W. Mott and J. Thompson, Metal Treatment, vol. 14, p. 228, 1948.
- W. Rosenkrantz, Aluminum, vol. 36, p. 250, 1960.
- K. L. Dreyer and H. J. Seeman, Aluminum, vol. 26, p. 76, 1944.
- W. Feldman, Metalwirtschaft, vol. 20, p. 501, 1941.
- D. S. MacKenzie, in First International Non-Ferrous and Technology Conference, St. Louis, 1997.
- H. G. Petri, Aluminum, vol. 24, p. 385, 1942.
- F. Keller and R. H. Brown, ‘Diffusion in Alclad 24S-T Sheet,” Trans. AIME, vol. 156, pp. 377-386, 1944.
- J. E. Hatch, Aluminum: Properties and Physical Metallurgy, Metals Park, OH: American Society for Metals, 1984.
- M. Cook, J. Inst. Metal, vol. 79, p. 211, 1951.
- M. Cook, J. Inst. Metals, vol. 80, p. 449, 1952.
- M. Cook, Proc. Royal Soc., vol. 205A, p. 103, 1951.
- B. R. Strohmeier, “Surface Characterization of Aluminum Foil Annealed in the Presence of Ammonium Fluoborate,” App. Surf. Sci., vol. 40, pp. 249-263, 1989.
- P. T. Stroup, “Heat Treatment of Aluminous Metals.” US Patent 2092033, 7 September 1937.

























