
The heat treatment of steel alloys is required in almost every industry to acquire the necessary properties for a material given a particular application. These properties include hardness, yield strength, fatigue strength, machinability, weldability, etc., and dictate the selection of the heat-treatment process used. To achieve these requirements, many heat-treatment processes are aimed at producing a mixed microstructure. This includes a ferrite/martensite mixture for dual phase steel heat treatments and processing of TRIP steels to produce ferrite/bainite or bainite/martensite mixed microstructures, to name just a few.
Of these microstructures, the inclusion of ferrite helps improve machinability, ductility, impact strength, etc. and is becoming a popular processing option. The formation of ferrite involves the rejection of carbon into the surrounding austenite matrix, due to the low solubility limit of carbon in ferrite. Increasing the carbon in the surrounding austenite results in modified martensitic and diffusive transformation behavior. This article will examine, through the use of experimental data and modeling using the DANTE heat treatment simulation software, the effects of carbon rejection during ferrite formation on transformation behavior.
Figure 1 shows strain versus temperature test data gathered from dilatometry experiments conducted on a European-grade, low-alloy carbon steel. Only the cooling portion of the test is shown. The blue curve begins at the austenitization temperature and is quenched to room temperature to form a fully martensitic microstructure. The orange curve begins at the austenitization temperature and is quenched to 715°C and held for a short ammount of time and then quenched to room temperature, for a microstructure consisting of ferrite and martensite. The effect on the martensite starting temperature (MS), seen in Figure 1, is significant; the carbon rejected during the ferrite transformation lowered the MS by 100°C. The lowering of the MS locally can have significant effects on the final part hardness, distortion, and residual stress.
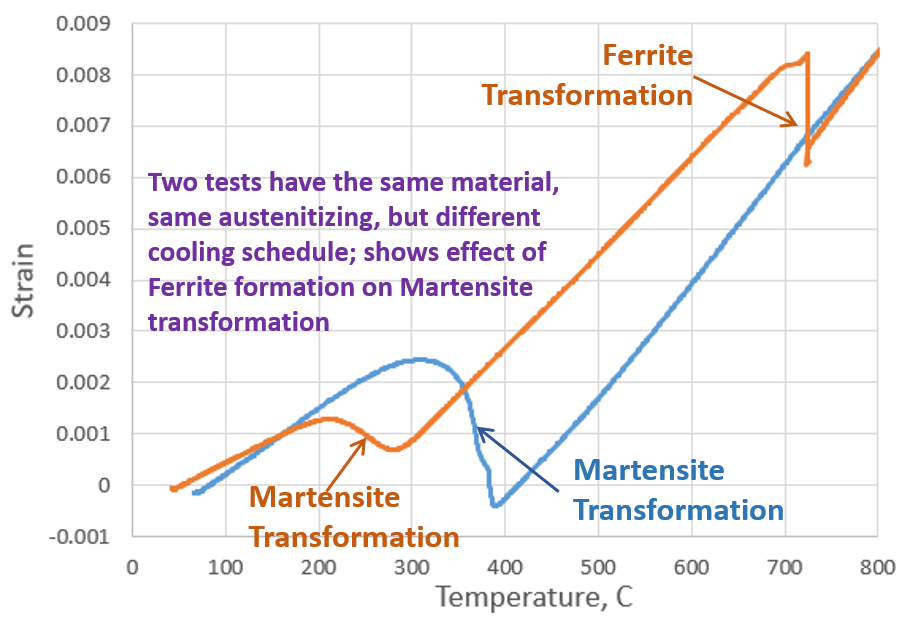
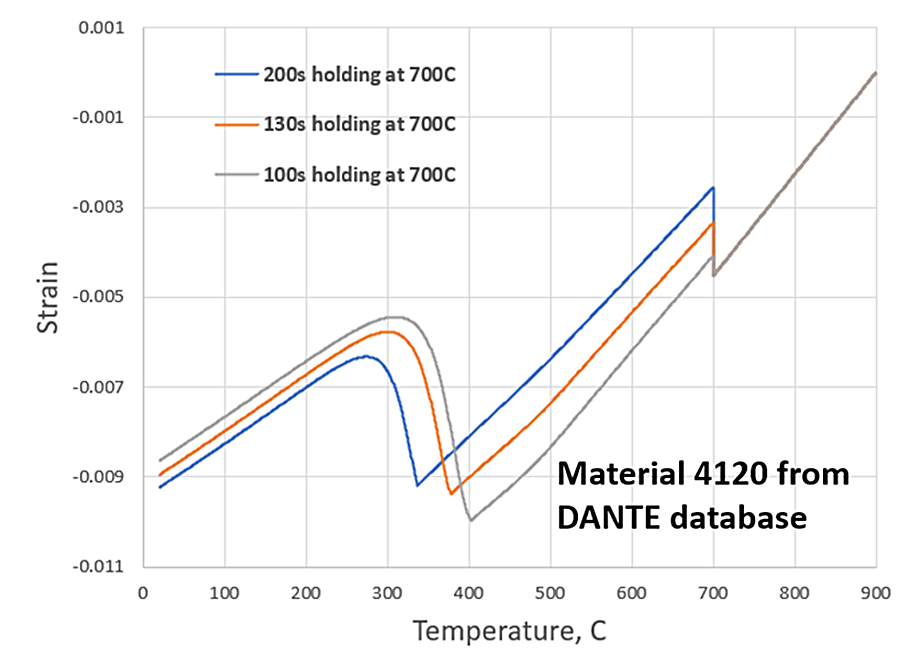
Figure 2 shows DANTE heat treatment simulation results for the steel alloy AISI 4120, which is available in the DANTE material database, quenched from the austenitizing temperature to 700°C, held for a prescribed amount of time, and then quenched to room temperature. The three holding times were chosen to produce a mixed microstructure consisting of ferrite and martensite. The 100-second hold produced 14 percent ferrite, the 130-second hold produced 33 percent ferrite, and the 200-second hold produced 49 percent ferrite; with martensite constituting the remaing phase. Figure 2 clearly shows the shift in MS due to the rejection of carbon during the partial ferrite transformation, with a lowering of the MS as more ferrite is formed and more carbon is added to the surrounding austenite matrix.
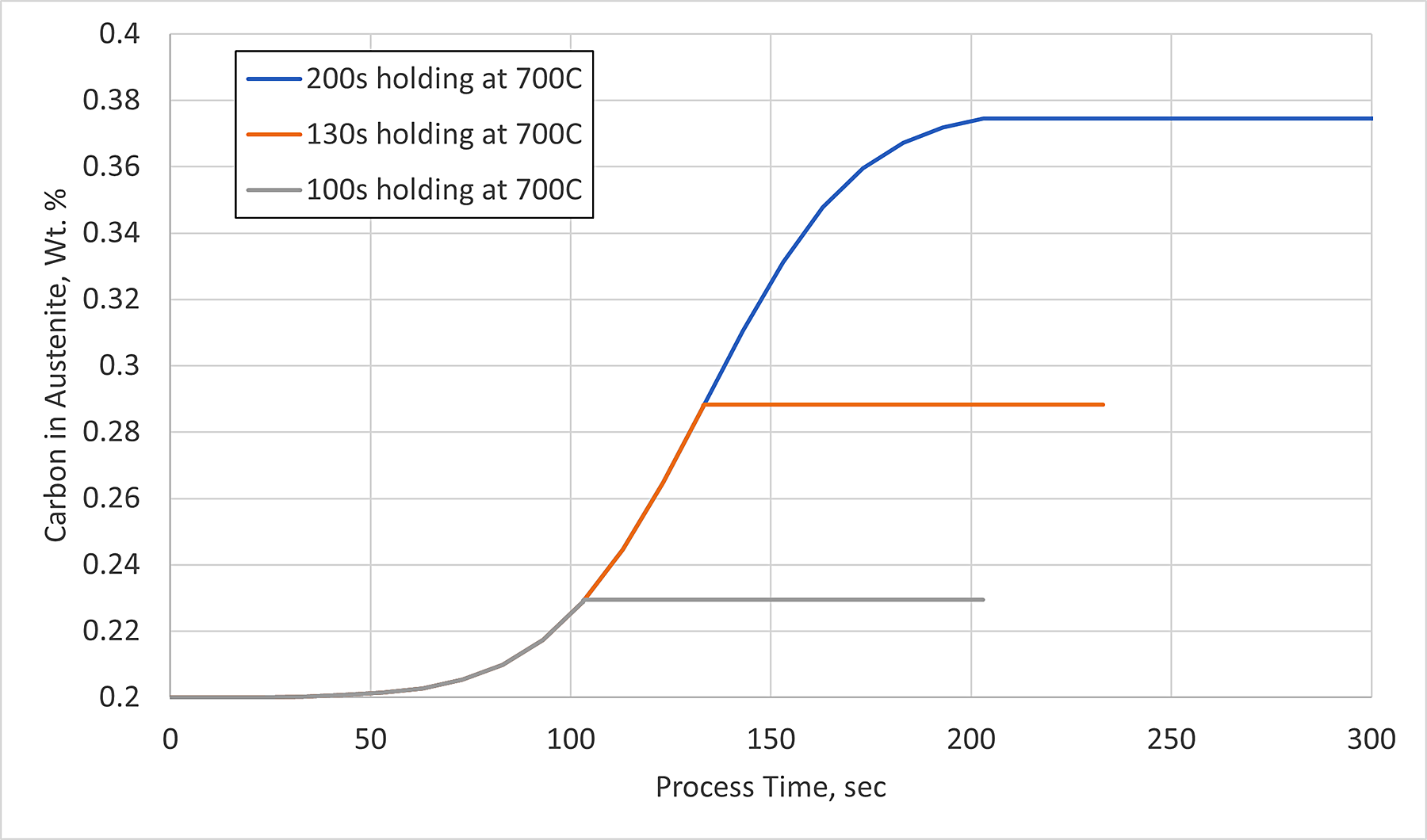
The carbon in the austenite matrix as a function of process time for the three simulated hold times are shown in Figure 3, with time zero equaling the start of quenching from the austenitizing temperature. Holding for 100 seconds, obtaining 14 percent ferrite, results in an additional 0.02 percent carbon in the surrounding austenite matrix. This is an insignificant increase and does not significantly affect the MS or diffusive transformation timing. However, when 49 percent ferrite is formed, by holding at 700°C for 200 seconds, the carbon in the surrounding austenite is essentially doubled, to 0.38 percent. Therefore, an increase in the carbon in the austenite matrix from 0.2 percent to 0.38 percent resulted in a reduction of the MS by 75°C, which can be significant if processing times and temperatures are based on the original carbon available.
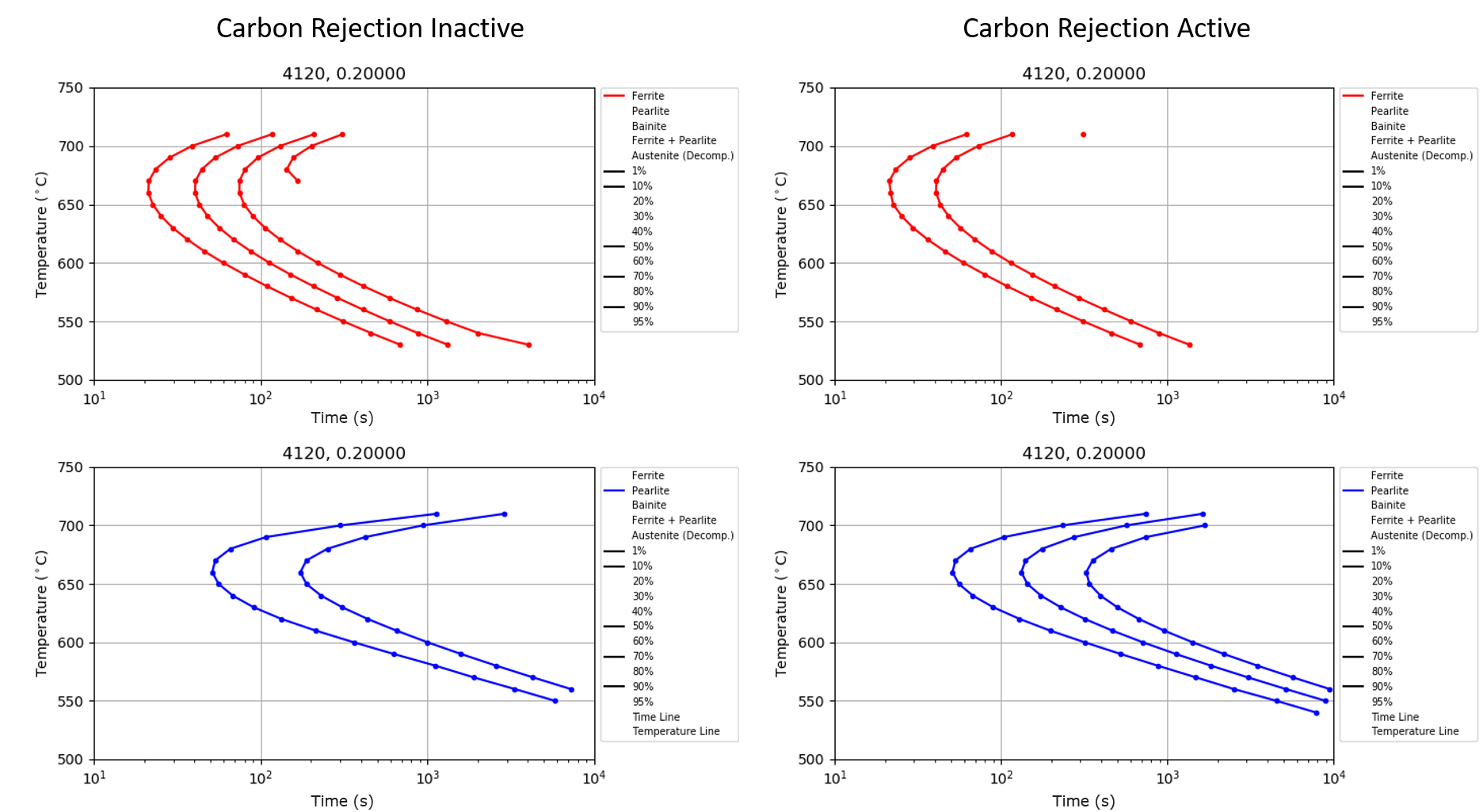
The previous discussion only examined the effect of carbon rejection from ferrite formation on the martensitic transformation. However, the rejection of carbon also influences pearlite formation and futher ferrite formation. Figure 4 shows TTT diagrams generated using DANTE’s TTT-Generator software and using AISI 4120 data contained in the DANTE material database. Figure 4’s left side shows the TTT diagram for ferrite (top left) and pearlite (bottom left) for AISI 4120 if carbon rejection during ferrite formation is ignored. Figure 4’s right side shows the TTT diagram for ferrite (top right) and pearlite (bottom right) for AISI 4120 if carbon rejection during ferrite formation is considered. Figure 4 clearly shows that if the rejection of carbon is ignored, more ferrite can be formed over a shorter time, forming 70 percent at 700°C. With more ferrite formed, there is less austenite available for the pearlite transformation, resulting in a reduced amount of pearlite, 10 percent at 700°C, when compared to the TTT diagram in which carbon rejection was condisdered. By contrast, considering carbon rejection, 10 percent ferrite and 50 percent pearlite is formed at 700°C. This can have an effect on the final hardness of the component.
Another way to view the effect of carbon rejection on the ferrite/pearlite transformation is to examine the ferrite and pearlite formed for a given process at a given point. Figure 5 shows DANTE’s Mat-Simulator software results for a process by which the material, AISI 4120, is quenched from the austenitizing temperature to 700°C and held for three hours and then quenched to room temperaure; only the first hour is shown since the transformations were already complete. The red lines represent ferrite, the blue lines represent pearlite, and the yellow lines represent austenite. The left side of Figure 5 shows that approximately 70 perecent ferrite is formed, with approximately 30 percent pearlite, if carbon rejection during the ferrite transformation is ignored. Accounting for the rejection of carbon from ferrite formation, shown on the right side of Figure 5, there are equal parts ferrite and pearlite. The difference in phase constituents will have an effect on the hardness of the component.
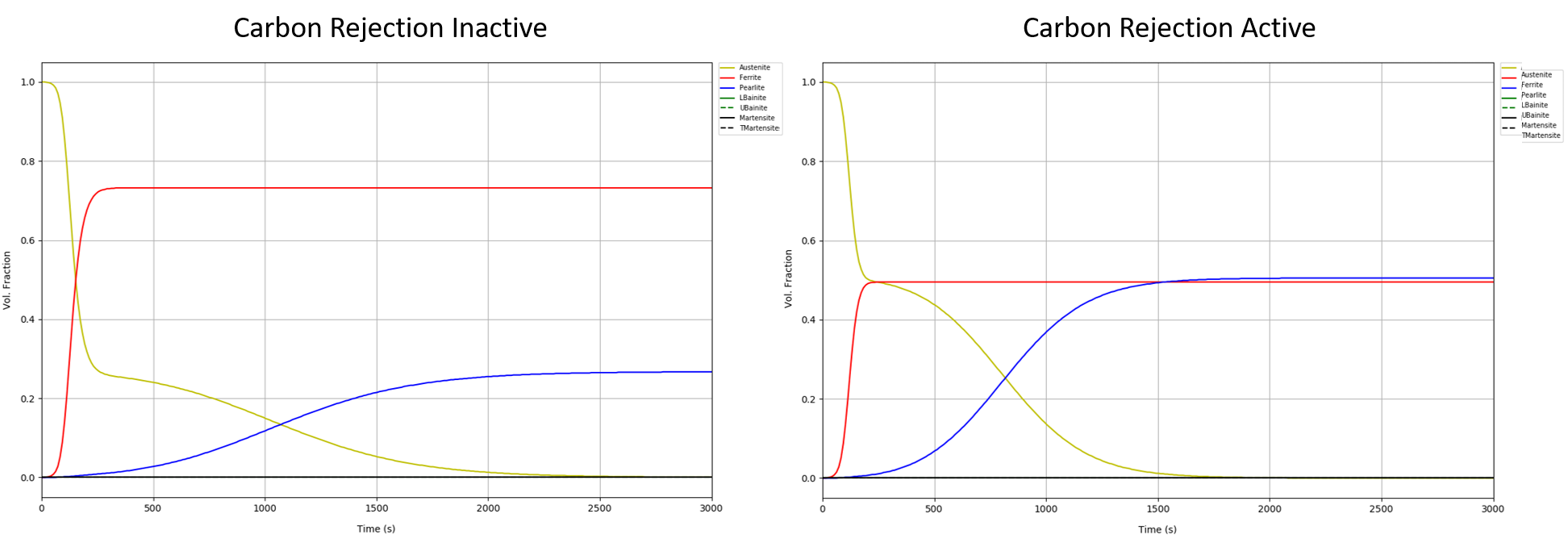
In conclusion, the rejection of carbon from the formation of ferrite should be considered in practice and in heat-treatment simulation. It has been shown, through experimental data and DANTE models, that the rejection of carbon from ferrite has a significant effect on the martensitic transformation, particularly the martensite starting temperature. DANTE models were also used to explore the effects of carbon rejection on the ferrite and pearlite transformations. It was shown that by accounting for the rejection of carbon, less ferrite, and more pearlite, can be formed for a given isothermal process. The local increase in carbon resulting from the rejection of carbon as ferrite is formed can lead to locally varying hardness and mechanical properties, ultimately affecting the final performance of the component. With the ability of the DANTE heat-treatment software to account for the rejection of carbon, simulation can now be used to evaluate this effect on final properties and help engineers and designers avoid post-production issues.


























