
In this column, I will discuss vapor pressure of mineral oils, and how to calculate the vapor pressure as a function of temperature.
Introduction
As the use of vacuum furnaces increases, there is also an increase in the number of vacuum integral quenches for leaner alloys. These furnaces process parts that, because of size or alloy hardenability, cannot be quenched using pressurized gases, such as helium and argon.
To properly maintain a vacuum in the furnace, the vapor pressure of the oil becomes critically important. An excessive vapor pressure can lead to oil depositing on interior furnace walls and vacuum pumps. A high vapor pressure will also contaminate the furnace vacuum. Therefore, it is necessary to be able to obtain the vacuum pressure of an oil as a function of temperature.
Clausius-Clapeyron Equation
As a liquid is heated, the vapor pressure steadily increases as the temperature increases. The Clausius-Clapeyron equation enables estimation of the vapor pressure (P2, atm) at another temperature (T2, °K), if the vapor pressure (P1, atm) is known at some temperature (T1,°K), and if the enthalpy of vaporization (ΔHvap) is known:
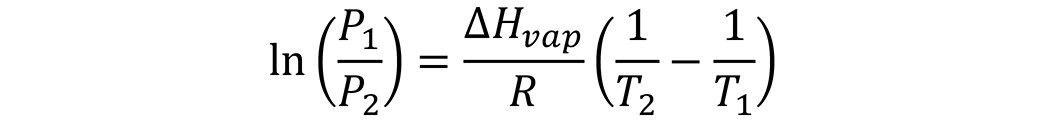 where R is the gas constant (8.3145 J mol-1 K-1).
where R is the gas constant (8.3145 J mol-1 K-1).
One common starting temperature that is used is the boiling temperature of the liquid (Tb). At this temperature, the vapor pressure is 1 atm. This greatly simplifies the math.
The last bit of information remaining is the determination of the enthalpy of vaporization (ΔHvap). From an early text by the Bureau of Standards [1], the enthalpy of vaporization for mineral oils was calculated from the equation:
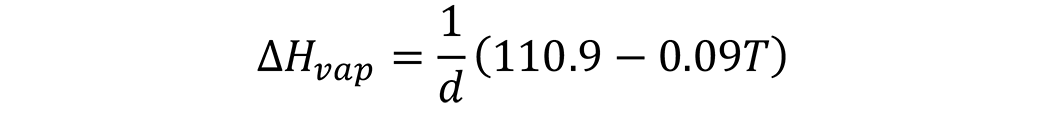 in which the enthalpy of vaporization is calculated in BTU/lb, d is the specific gravity of the liquid at 60°F, and T is the temperature (°F). However, the enthalpy of vaporization is given in J mol-1, so some unit conversion is necessary. To convert BTU/lb to J/Kg, the BTU/lb is multiplied by 2,326 to yield J/kg. However, the units are J/mol, so additional calculation is needed. The molecular weight of the oil is needed.
in which the enthalpy of vaporization is calculated in BTU/lb, d is the specific gravity of the liquid at 60°F, and T is the temperature (°F). However, the enthalpy of vaporization is given in J mol-1, so some unit conversion is necessary. To convert BTU/lb to J/Kg, the BTU/lb is multiplied by 2,326 to yield J/kg. However, the units are J/mol, so additional calculation is needed. The molecular weight of the oil is needed.
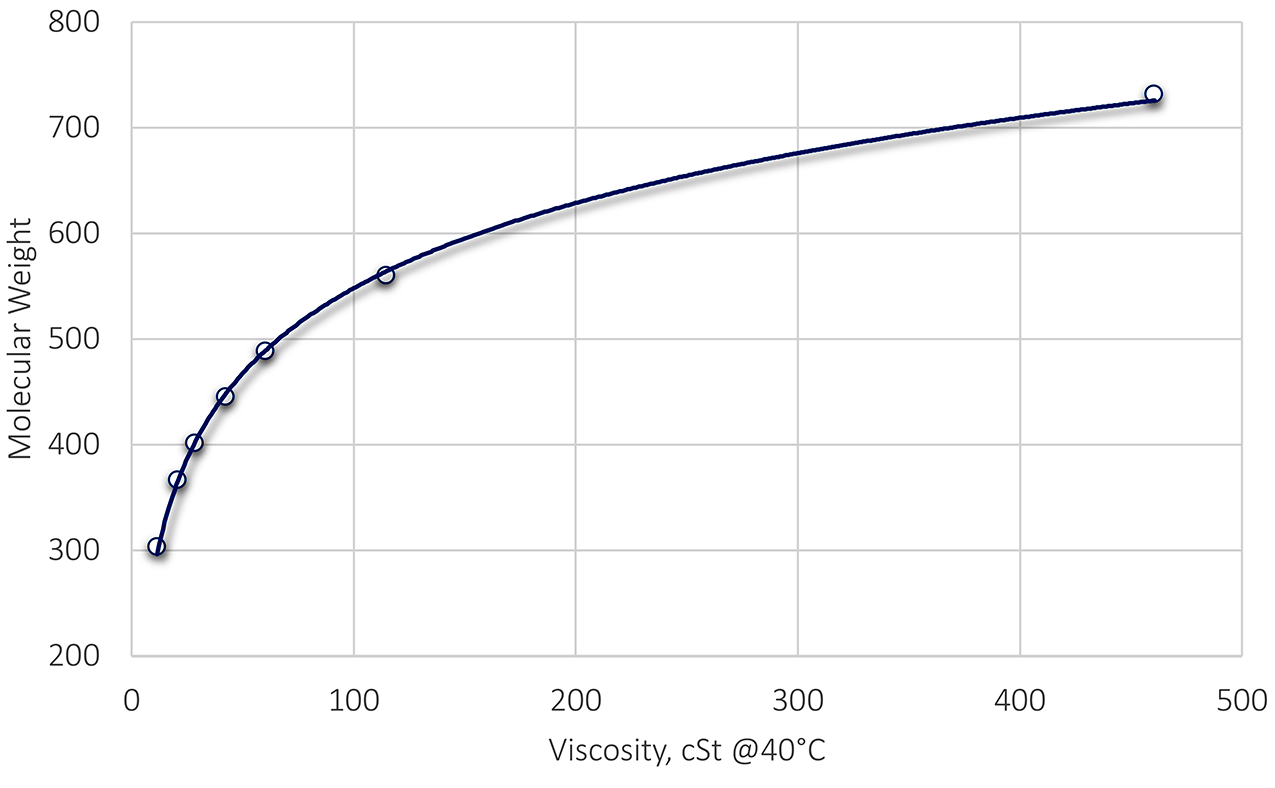
The molecular weight of the oil may be provided in the technical data sheet. However, if not, an additional relationship is needed. A common relationship between the viscosity of an oil (cSt @ 40°C) and the molecular weight of straight cuts of oil is shown in Figure 1. This relationship is:
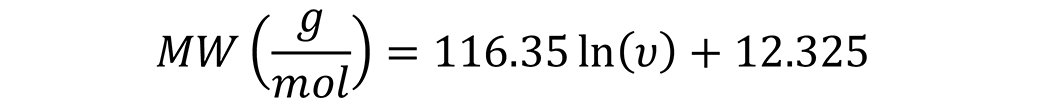 For mixtures of different straight cuts of oil to obtain different viscosities, a different, much more complex method is required [2]. In general, mixtures to obtain a specific viscosity, will have a higher vapor pressure than will a straight cut or single viscosity.
For mixtures of different straight cuts of oil to obtain different viscosities, a different, much more complex method is required [2]. In general, mixtures to obtain a specific viscosity, will have a higher vapor pressure than will a straight cut or single viscosity.
To convert J/Kg to J/mol, the enthalpy of vaporization in J/Kg is multiplied by the molecular weight (g/mol) divided by 1000 to obtain Kg/mol:
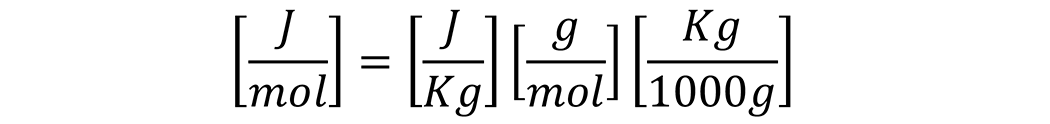 The enthalpy of vaporization in J/mol is then obtained.
The enthalpy of vaporization in J/mol is then obtained.
An alternative method [3] of calculating the enthalpy of vaporization is based on the boiling temperature of the oil, in °K [4]:
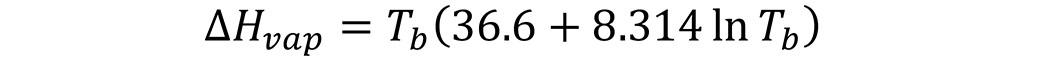 where Tb is the boiling temperature of the liquid, °K. The boiling temperature of the liquid can usually be found in the Safety Data Sheet of the product under “Physical and Chemical Properties”. This is a much easier and simpler method as it does not require conversion of units or knowing the molecular weight of the product. The two methods correspond within 5 percent to each other. Other methods are also presented in [3].
where Tb is the boiling temperature of the liquid, °K. The boiling temperature of the liquid can usually be found in the Safety Data Sheet of the product under “Physical and Chemical Properties”. This is a much easier and simpler method as it does not require conversion of units or knowing the molecular weight of the product. The two methods correspond within 5 percent to each other. Other methods are also presented in [3].
Determination of vapor pressure
Using typical data obtained from the technical data sheets and the Safety Data Sheets of the different viscosity oils (Table 1), the vapor pressure was determined as a function of temperature up to the flash point for these oils using the Clausius-Clapeyron Equation. ΔHvap was calculated from the method of Zhao and Yalkowsky [4]. Boiling temperatures and specific gravity were obtained from the SDS of each product. Temperatures were all converted to °K. Data was converted to Torr from atm (1 atm = 760 mm Torr). The resulting data is shown in Figure 2.
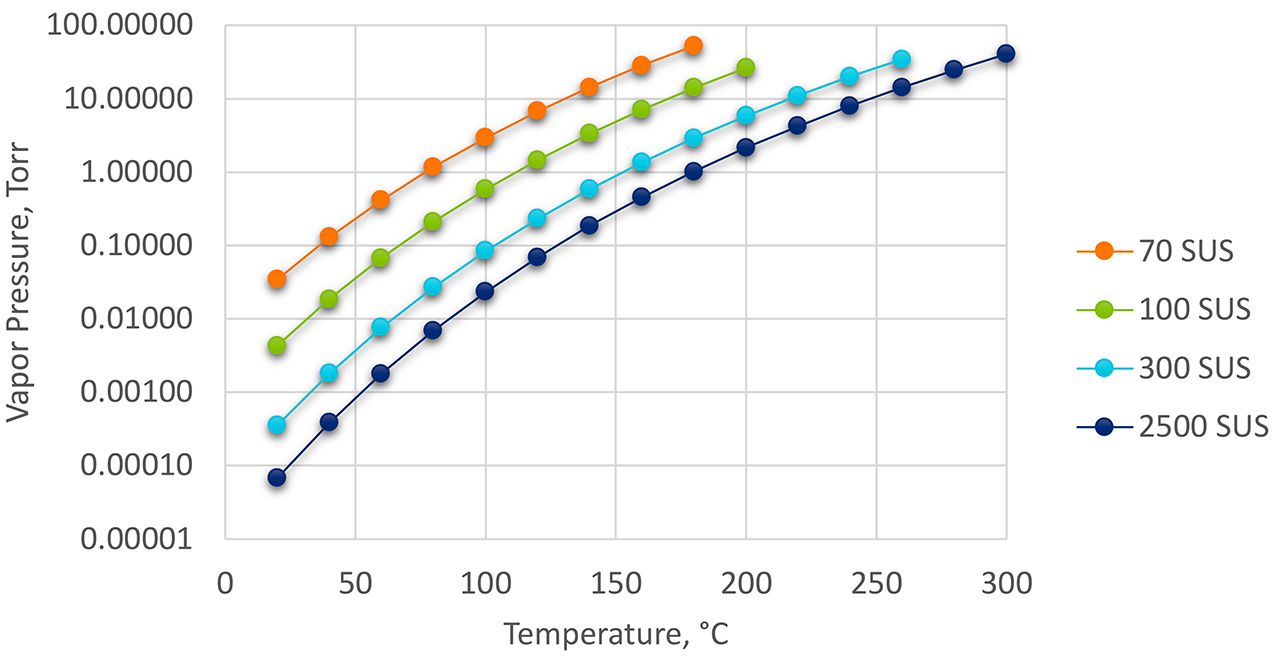
From the calculated data, the effect of viscosity and molecular weight is apparent. As the viscosity (and molecular weight) increases, the vapor pressure decreases at the same temperature. In other words, it is desired to use the highest viscosity possible that will still achieve as-quenched properties. Comparing two oils that have the same quenching characteristics, the one with the highest viscosity will have the lower vapor pressure, requiring a reduced atmosphere backfill. Further, as temperature increases, the higher viscosity oil will have the lowest vapor pressure.
 Conclusions
Conclusions
In this article, a method to estimate the vapor pressure of straight cut mineral oils as a function of temperature was provided, using the Clausius-Clapeyron Equation. Further, two methods of calculating the enthalpy of vaporization, ΔHvap was illustrated. While mixtures of oils to achieve a specific viscosity was not covered in this article, further reading of the references will help understand the vapor pressure of mixtures. In general, mixtures to obtain a specific viscosity will have a higher vapor pressure than will a straight cut or single viscosity.
Should there be any questions or comments regarding this article, or suggestions for further columns, please contact the writer or editor.
References
- The Bureau of Standards, “Thermal Properties of Petroleum Products,” U.S. Department of Commerce, Washington D. C., 1929.
- D. L. Katz and G. G. Brown, “Vapor Pressure and Vaporization of Petroleum Fractions,” Industrial and Engineering Chemistry, vol. 25, no. 12, pp. 1373-1384, 1933.
- W. Fang, Q. Lei and R. Lin, “Enthalpies of vaporization of petroleum fractions from vapor pressure measurements and their correlation along with pure hydrocarbons,” Fluid Phase Equilibria, vol. 205, pp. 149-161, 2003.
- L. Zhao and S. H. Yalkowsky, “A modification of Trouton’s rule by simple molecular parameters for hydrocarbon compounds,” Ind. & Eng. Chem. Res., vol. 38, no. 1, pp. 324-327, 1999.





















