
Last month, I began a two-part article about troubleshooting induction hardening problems and discussed possible corrections. This month, I will discuss biological and odor problems, and corrosion issues.
Induction hardening is a unique method used to harden steels. The process uses a power supply, RF generator, induction coil, and quenching mechanism (spray or immersion) to yield a high surface hardness and advantageous residual surface stresses.
There are many problems that can occur in induction hardening that can have nothing to do with the power supply, RF generator, or coil. These are process-related issues that can be traced back to improper or inadequate process control.
Biological and Odor Problems
Biological issues such as bacteria and fungi can be a major issue in induction hardening. Bacteria and fungi are floating in the air. When they land in a moist place they tend to grow. Fungus tends to grow at difficult to reach places where there is little motion of the fluid. Oftentimes this is at the waterline of the quench tank. Bacteria likes to grow in stagnant, oxygen-depleted, food-rich locations. Scale and other debris are wonderful food for anaerobic bacteria found in the typical quench tank. Bacteria and fungi can change the cooling curve of the polymer. This is done by either degradation of the polymer, or by other mechanisms [1]. In addition to changes to the cooling curve of the quenchant, they can produce very unpleasant odors – such as a rotten egg smell, or a badly mildewed locker room. Figure 1 shows an extreme example of bacteria and fungi grown on a dip slide after 24 hours. Additionally, the presence of bacteria and fungi lower the pH, which can increase problems with corrosion or rusting.
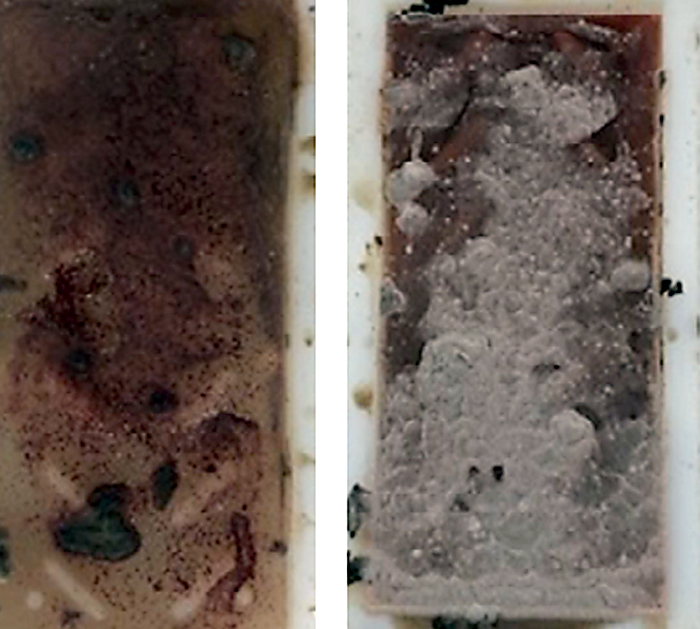
Cleanliness and oxygen are important controlling factors to minimize the occurrence of bacteria. Keeping the quenchant moving will minimize the occurrence of bacteria. Motion of the quenchant will also entrain air, which helps minimize bacteria. However, excessive agitation can result in splashing. This splashing can settle on difficult-to-reach locations where fungus can grow.
Contamination from outside sources is often a prime driver for bacteria and fungus growth in a polymer quench tank. Machining coolants are often not cleaned from parts prior to induction hardening. They often contain large quantities of swarf, bacteria, and fungi. This is bad practice. Quench tanks are not parts washers — they collect everything (Figure 2). Make sure that skimmers, agitators, and associated equipment is working properly. Coolant should be removed from parts by proper cleaning and rinsing prior to induction hardening. For instance, if a part contains just 5 ml of coolant (one teaspoon), and there are 3,000 parts produced per day, this means that 15 liters of contaminant are dragged into the typical 500-liter quench tank per day. It doesn’t take long before the quench tank containing only water and polymer becomes a coolant contaminated mess (Figure 3).


To control biological contamination, biocides are often used to kill bacteria and fungi [2]. These biocides are used to control and kill unwanted microorganisms. Not only do biocides kill pathogens, they also kill non-pathogens. This means that they can be dangerous to humans. Only tiny amounts are necessary to kill the bacteria and fungi present. For a typical 500-liter quench tank, only 1.25 fluid ounces are required to kill the bacteria and fungi. Biocides are severe skin irritants and corrosive to eyes. They may also cause burning of the skin and cause skin sensitization (contact dermatitis) [3]. Proper personal protection equipment, including face shields, goggles, rubber gloves, protective suits, and other PPE are necessary to prevent personnel injury. Not only are biocides hazardous, they are also very expensive.
Biocides only last for 2 to 4 weeks depending on the contamination, and the number of new bacteria and fungi brought into the system. Constant dosage of new biocide may initially keep bacteria and fungi low, but eventually, resistant bacteria and fungi will be created. Generally, anything that the heat treater can do to avoid using biocides is a good idea.
Alternatively, one can use specially developed bio-resistant polymer quenchants. These quenchants are specially formulated to be resistant to biologicals, and not create the odors associated with biologicals. A limited or specialized biocide may be present, depending on the quenchant. These quenchant will minimize the use of biocides and be damage tolerant to contamination.
Corrosion Issues
Corrosion issues can be the result of a depletion of corrosion inhibitors, or biological contamination. It can be the result of improper washing and rinsing of the parts after induction heat treatment.
Most polymer quenchants have both ferrous and non-ferrous corrosion inhibitors. They serve several purposes. The first is to protect the machine. Most induction hardening equipment uses a variety of different materials – copper, aluminum, steel, etc. This is wetted by the quenchant and so galvanic corrosion is a real problem to be overcome. Stray currents and improperly grounded equipment can make corrosion issues worse. Secondly, the hardened part needs short time corrosion protection.
Corrosion from chlorides can be a real problem. Most tap waters contain nominal amounts of chloride as part of water treatment facilities by cities. If a well is used, the chloride level in the water can peak in April and May due to the infiltration of chlorides from road salt. As equipment is used, water evaporates. The inorganic load in the equipment does not evaporate but builds concentration. Once this concentration exceeds some chloride value, corrosion in the form of flash rusting can occur. This value of chloride varies by customer and practice. A concentration greater than 25-50 ppm chloride is considered the threshold for corrosion.
If corrosion is an issue, the supplier may be able to supply an additive package to enhance the corrosion protection of the quenchant. Additionally, washing (and rinsing) any residual polymer from the part is recommended. If the parts are not properly washed (and rinsed), or if the parts are not washed at all, there will be residual polymer and water film remaining on the part. During tempering, water will be driven off, and will concentrate any residual chlorides and will become a source for flash rusting. Washer residues from alkaline cleaners can also be a source for corrosion. It is extremely important that parts are properly rinsed to avoid residues that can cause corrosion to the active hardened surface.
Conclusion
In this article we have tried to provide an understanding of the potential for problems during induction hardening, and to provide the user with some possible corrective actions. Many of the issues in induction hardening can be directly related to the amount of contamination in the quench system, and improper or inadequate cleaning.
Should you have any questions regarding this article, or have suggestions for new articles, please contact the editor or the author.
References
- J. Barberi, C. Faulkner and D. S. MacKenzie, “Extending the Life of Polymer Quenchants: Cause and Effect of Microbiological Issues,” in Proceedings from the 6th International Quenching and Control of Distortion Conference, 09-12 September, Chicago, 2012.
- J. L. Shennan, “Selection and evaluation of biocides for aqueous metal-working fluids,” Tribology International, vol. 16, no. 6, pp. 317-330, 1983.
- Rohm and Haas, “KATHON™ 886 MW Microbicide,” Rohm and Haas, 2006.






















