Nitriding is the thermochemical process for producing surface layers in ferrous alloys, such as steels and cast irons used for making various mechanical components and tools [1]. Ferritic nitrocarburizing (FNC) is a process of nitriding doped with a small amount of carbon [1]. Producing the best nitrided layers for the given application requires a good cooperation between designers of the product and the manufacturing companies making it. Nitriding is a type of heat treating, which has a significant effect on the final properties of the product in improving its tribological properties. It reduces friction and enhances wear resistance of the surface as well as produces very good bending fatigue and corrosion resistance properties. All the above is decided by formation of the proper structure, thickness, and hardness of the nitrided layer. Metallography of the parts, or samples which run together with them, is extremely important for verifying results of this thermochemical treatment and assessing the properties of the layer formed, the data are also used for maintaining a good predictability of the process. It should be noted; however, that surface condition of machined parts may significantly affect the nitriding outcome and should always be taken under consideration [2-4].
Metallographic Characteristics of Nitrided Layer
Low-alloy steels
The typical nitrided layer formed in these steels has two sub-layers: the compound zone and diffusion zone. See Figure 1a and 1b. The compound zone increases wear and corrosion resistances, and the diffusion zone enhances the bending fatigue strength of the component.
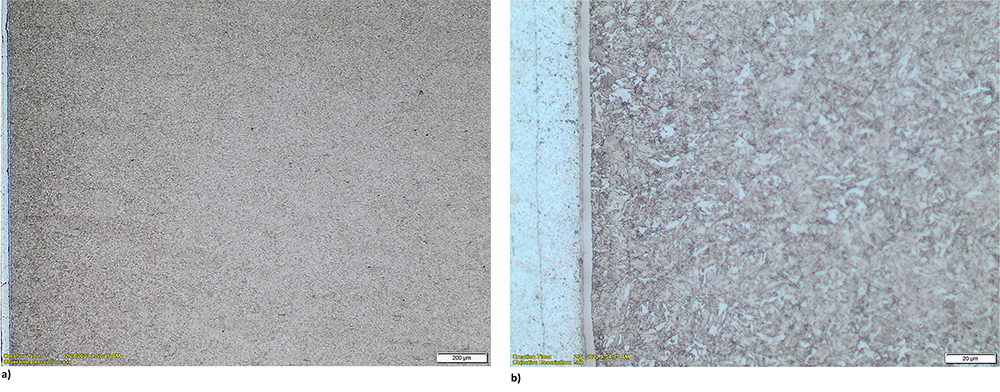
As it can be noticed, the diffusion layer cannot be easily seen under the microscope in the low-alloying steels such as 4340, 4140, and others. Therefore, it is very useful to measure microhardness of the sample on its cross section to get the information on how deep the diffusion of nitrogen really was and also the hardness of this layer. See Figure 2.
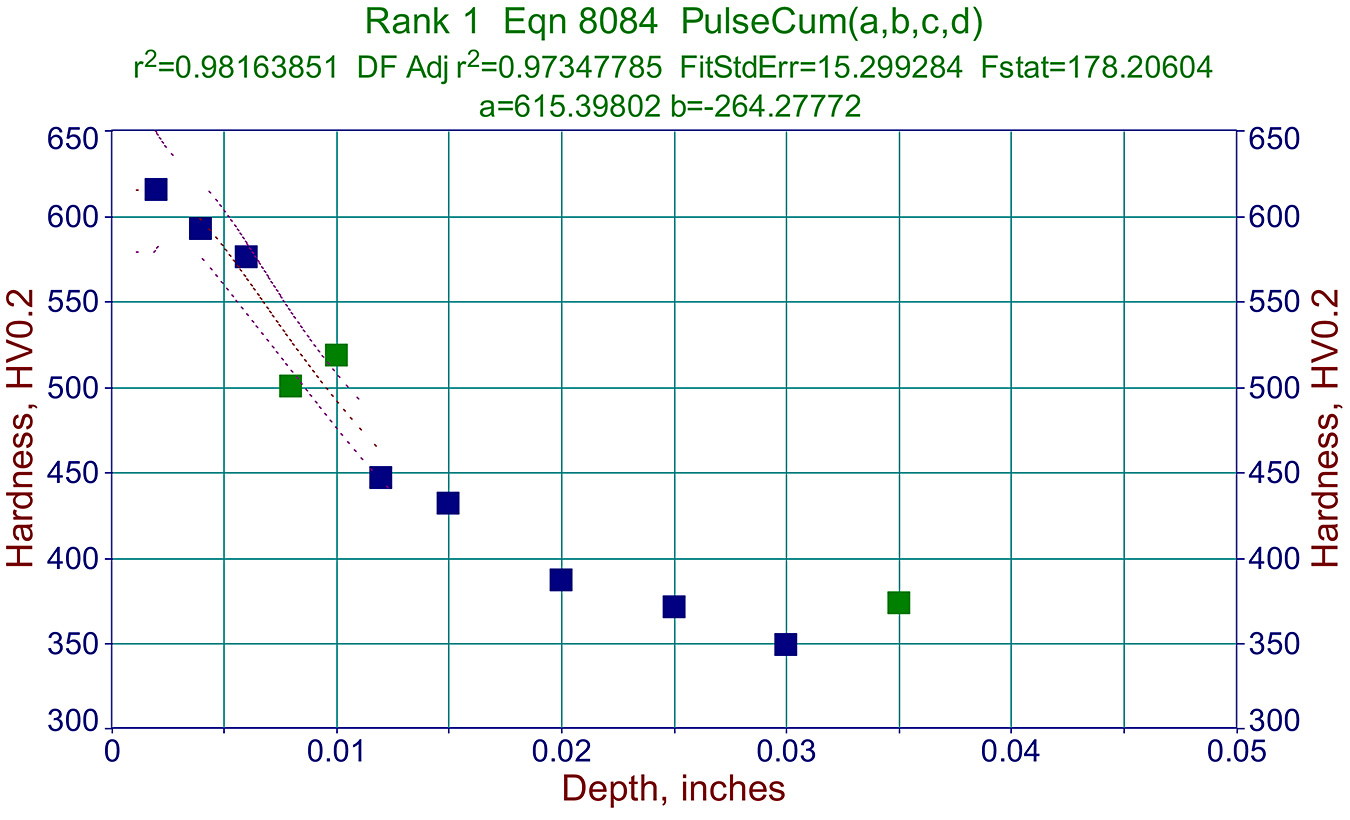
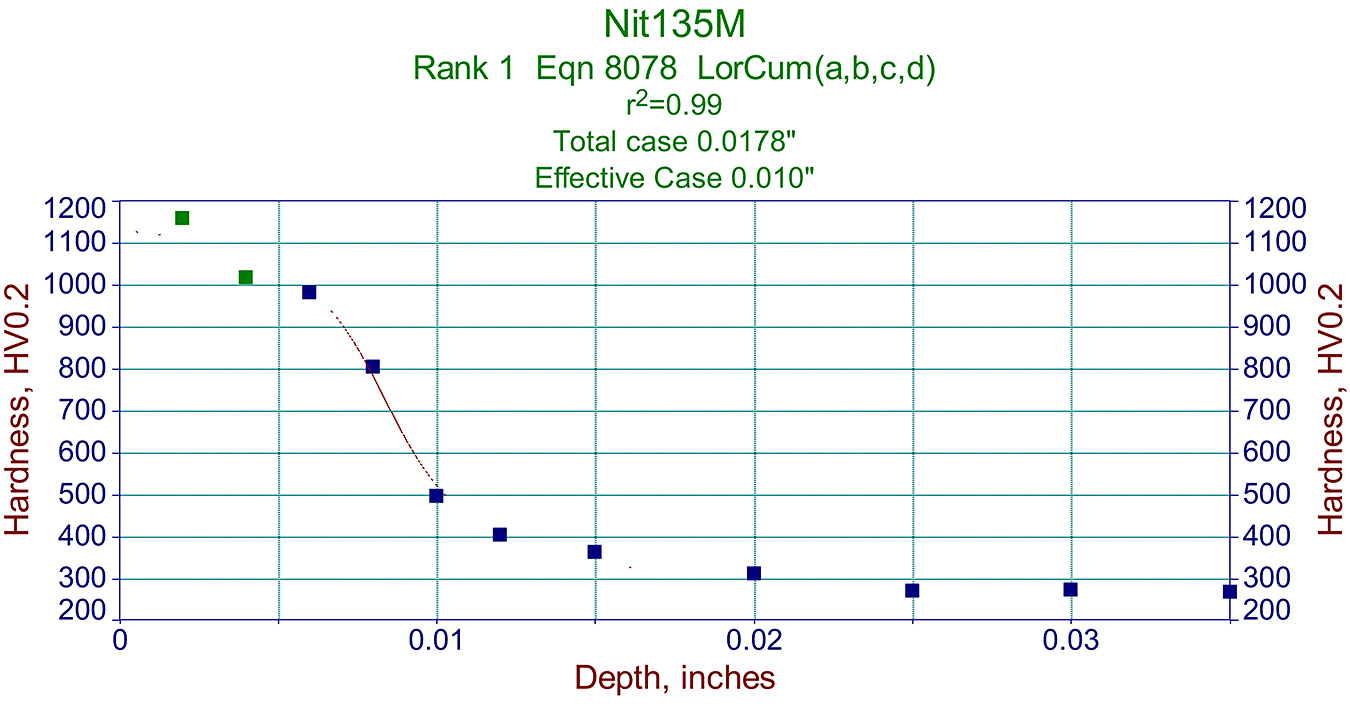
Metallographic data like that above, are also very useful in making proper graphs used for predicting a cycle required for specific components. See example in Figure 3.
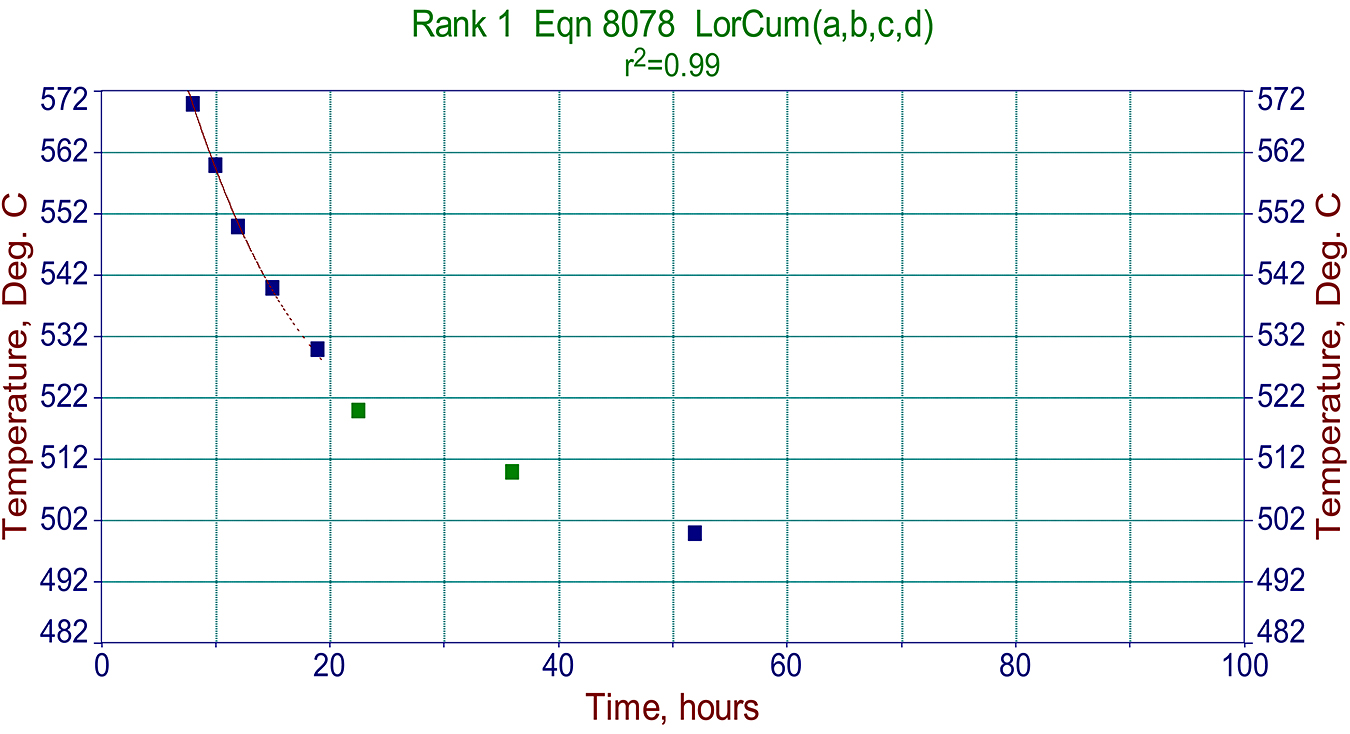
Data such as those presented above also can be used for making more sophisticated 3D graphs illustrating nitriding kinetics for various steels such Nit135M, 4140, and others. This information helps metallurgists at Advanced Heat Treat Corp. to write proper cycles for specific steels and the specific case depth requirements and properly set them for variously heat-treated steels with different core hardnesses. A typical kinetic graph is shown for Nit135M steel in Figure 4.
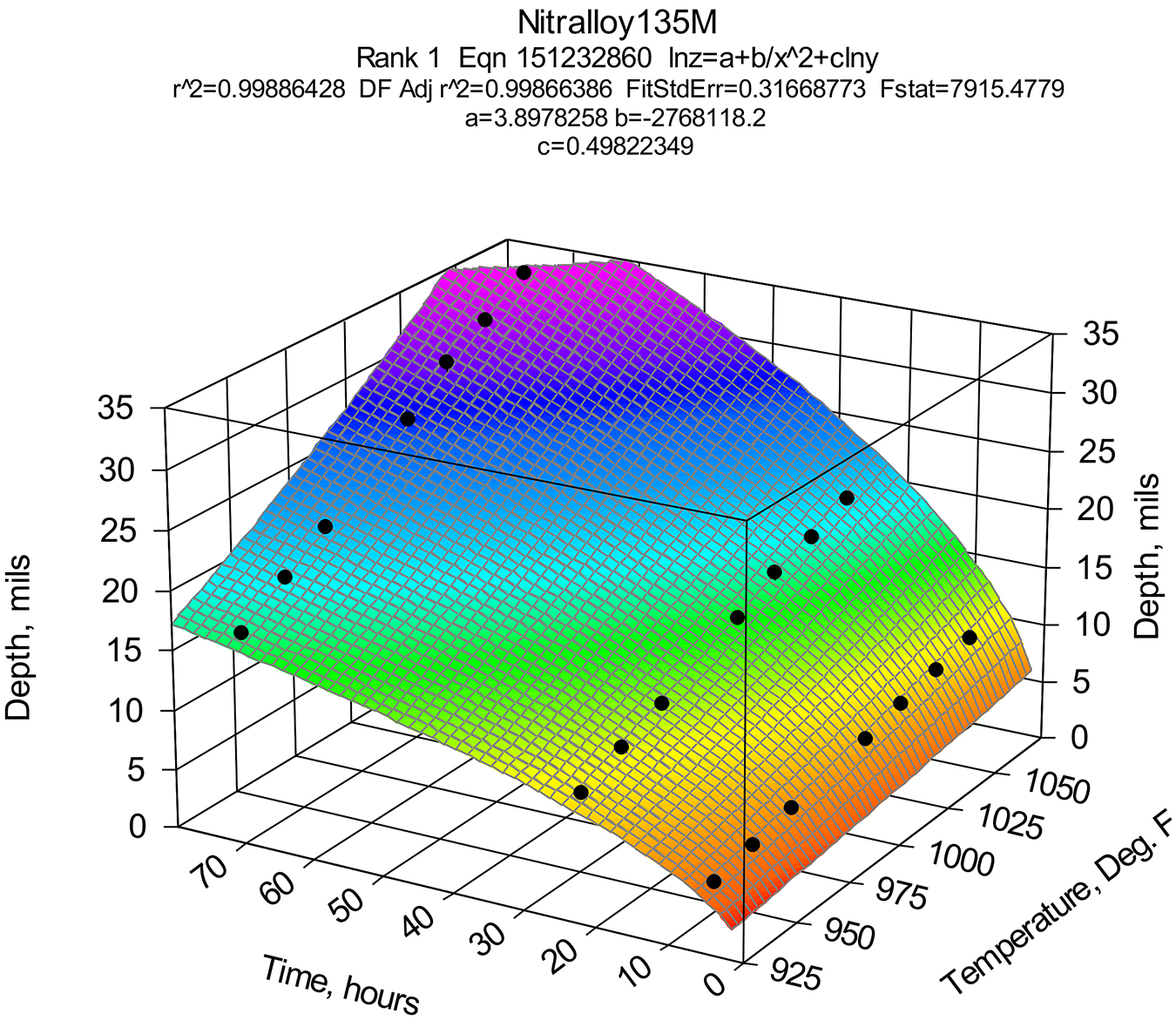
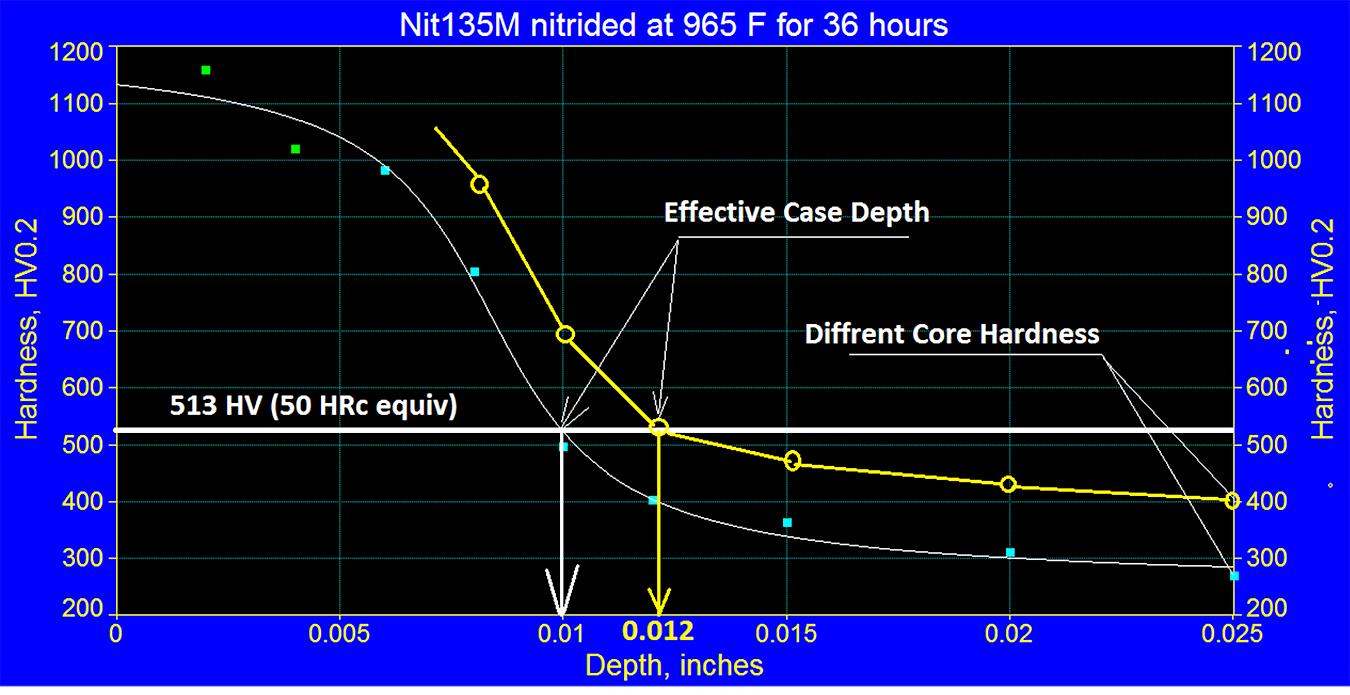
It should be noted that the effective case for this specific Nit135M steel was >50% of total case depth. See Figure 5.
However, it should be remembered that the relationship between the effective case depth’s percentage of the total case varies depending on steel and its tempering temperature. Therefore, it is important to note the thickness of the effective case depth depends on the core hardness of the sample used for certifying the load, and it may be different than in the actual parts. As mentioned before, this depends on the tempering temperature of the steel. See Figure 6.
Plain carbon steels
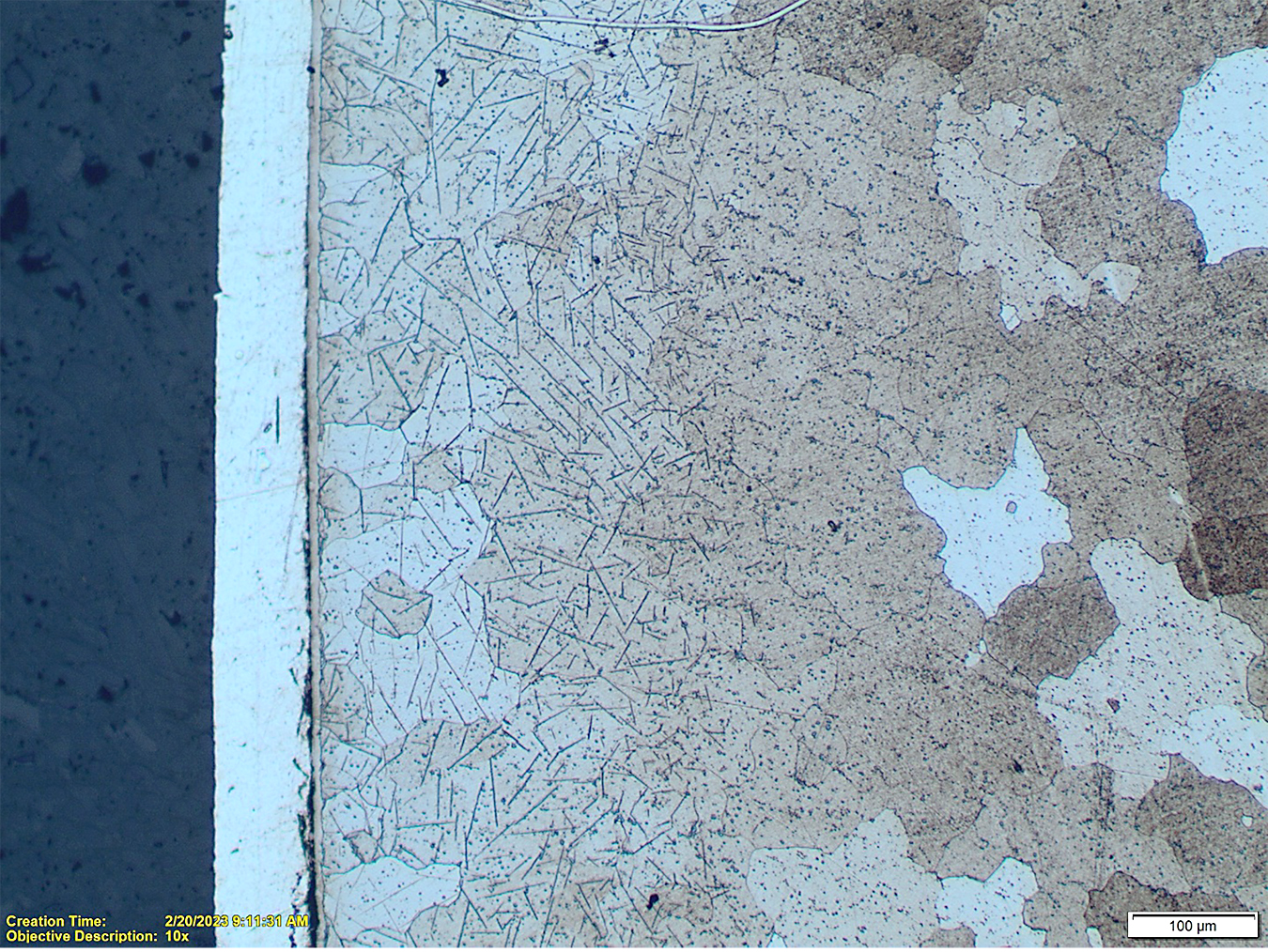
Nitriding of the plain carbon steels such as 10xx, 11xx, 12xx and other similar carbon steels is oriented on formation of the compound zones of sufficient, larger than in the low-alloy steels, thicknesses. This is caused by the fact these steels do not form sufficiently hard diffusion zones and also that their typical applications do not require a high bending fatigue strength but rather wear and corrosion resistance. There is a variety of the compound zone structures formed in these steels. Some of the examples are shown in Figures 7 and 8.
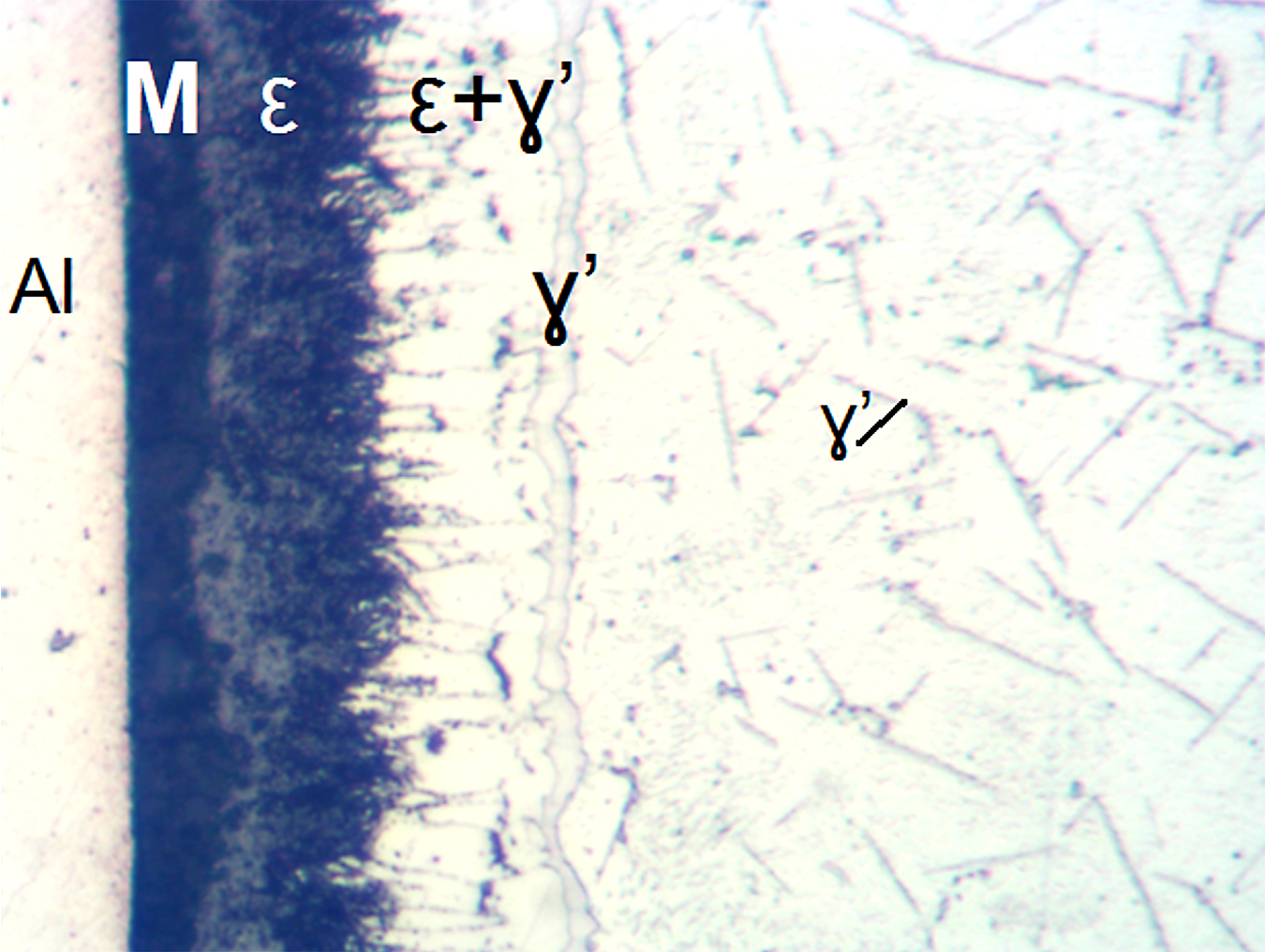
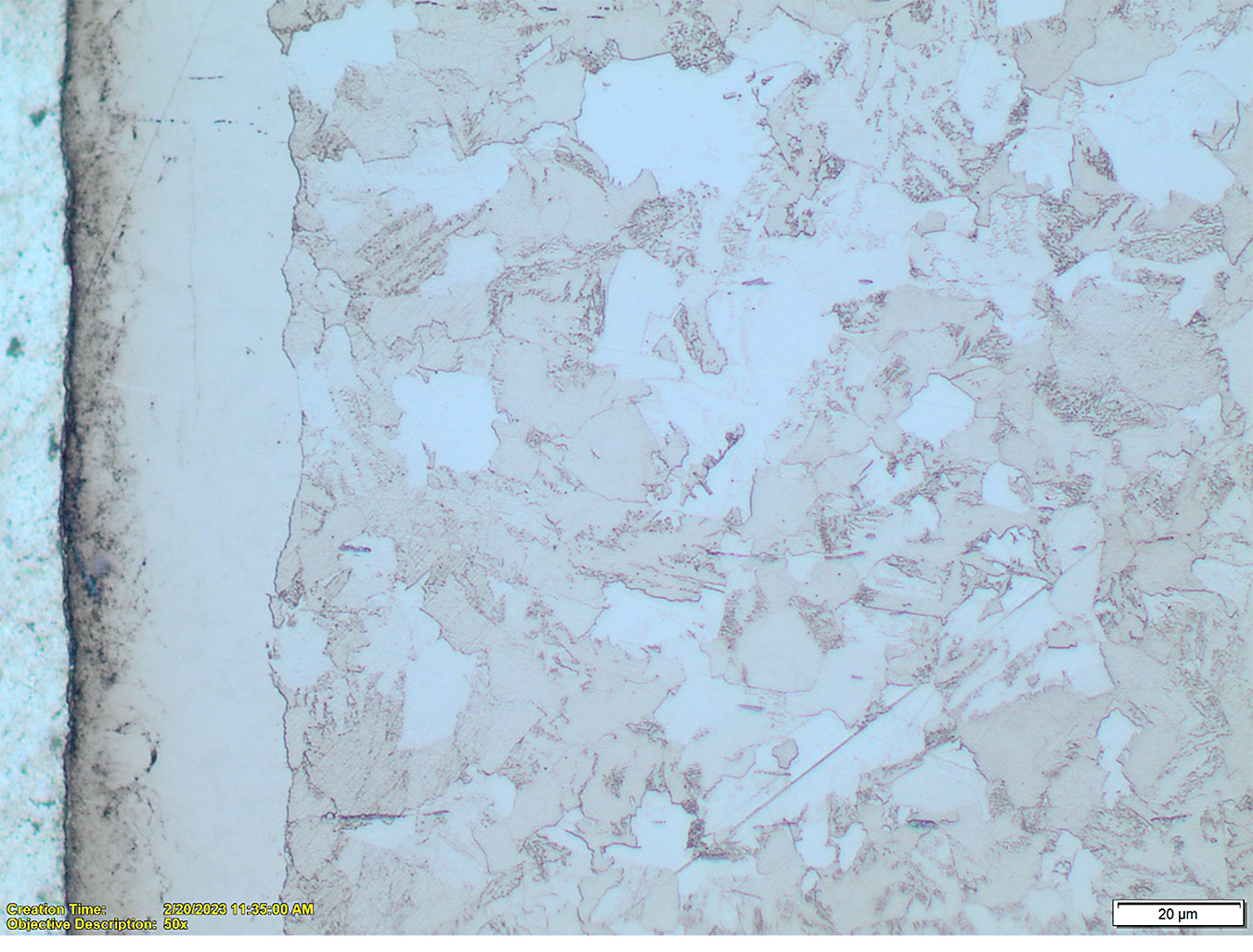
It also should be noted the total thickness of the nitrided layers in these steels can be assessed by microscopic evaluations without a need for the microhardness testing. Diffusion layer in the carbon steels has typically precipitates of the gamma prime, γ’ (Fe4N) or Fe16N2 nitrides visible in the structure, see Figures 9 and 10.
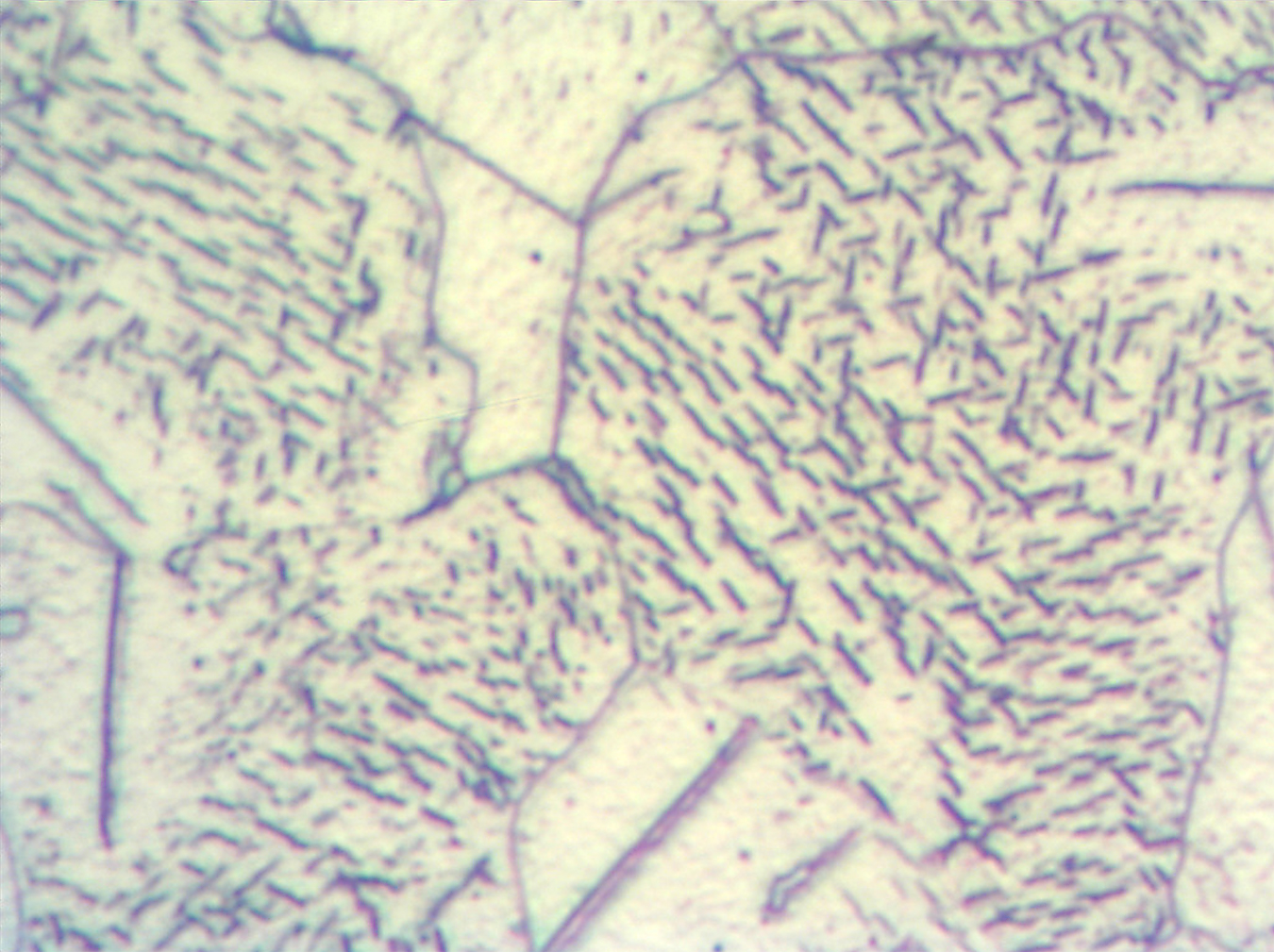
However, those nitrides might not be visible if a cooling applied after nitriding was sufficiently fast to cause supersaturation of ferrite.
Identification of some of the surface defects affecting nitriding
Surface condition of steel parts has tremendous effect on their ability to accept thermochemical treatment such as nitriding or nitrocarburizing [1, 2, 3]. Too aggressive machining leading to distortion of the steel structure, its cold work, burnishing, or inducing tensile stress at the surface may completely stop or inhibit nucleation of nitrides at the surface [1-4]. The same, negative effect on nitriding can be expected from surface contaminations if the metalworking fluids and coolants are not completely removed from prior nitriding or FNC [2-4]. On the other hand, compressive stress induced by machining or shot peening into the surface may have a very positive effect on formation of the nitrided layer [4].
 The risk of producing unwanted stress condition by machining is very high, and, therefore, general industrial/heat-treating practice is to perform the stress relieving or recrystallize-anneal operation after rough, aggressive machining. After that, final machining should be applied to remove any remaining surface defects or conditions before nitriding or nitrocarburizing can be applied.
The risk of producing unwanted stress condition by machining is very high, and, therefore, general industrial/heat-treating practice is to perform the stress relieving or recrystallize-anneal operation after rough, aggressive machining. After that, final machining should be applied to remove any remaining surface defects or conditions before nitriding or nitrocarburizing can be applied.
Machining operation affects the surface structure of 12xx steel components resulting in distorted grains and a possible smeared surface. Insufficient stress relief/recrystallization after that may result in a condition that affects nitriding or FNC processes resulting in waviness as well as insufficient thickness of the compound zone. Also, effects of sulfides present in the steel cannot be overcome easily. Sulfides are a physical barrier to the diffusion of nitrogen into the steel and, if they are present near the surface (and they are), they reduce effectiveness of nitriding, see Figure 11. Such voids are very likely to occur since the sulfides are present everywhere, and they may locally stop or limit diffusion of nitrogen. These phenomena cannot be controlled by any action of the machinists or heat treaters. Potential defects and surface imperfections of the steels affecting formation of the nitrided layers can be metallographically discovered and analyzed. See an example in Figures 11 and 12.
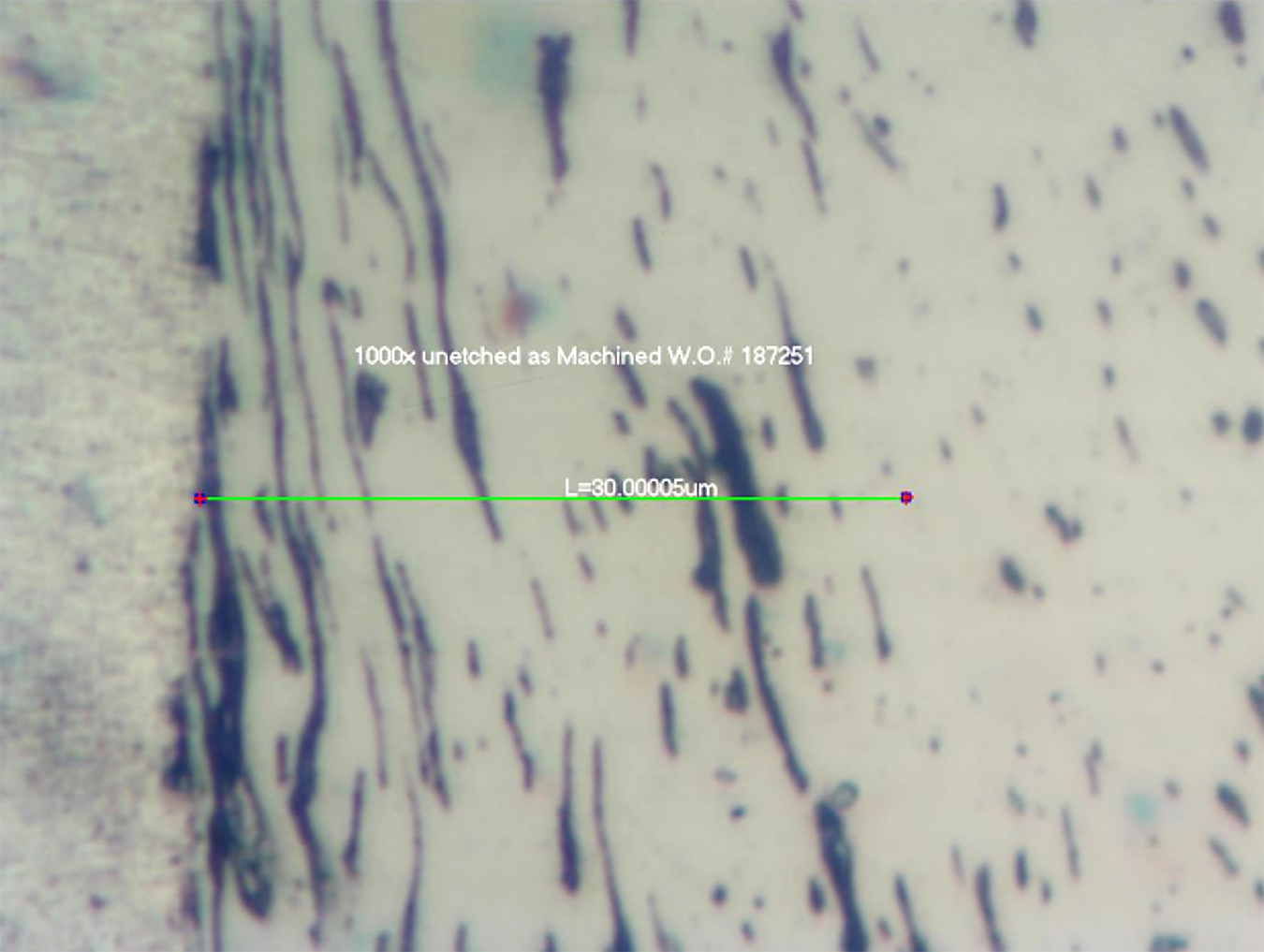
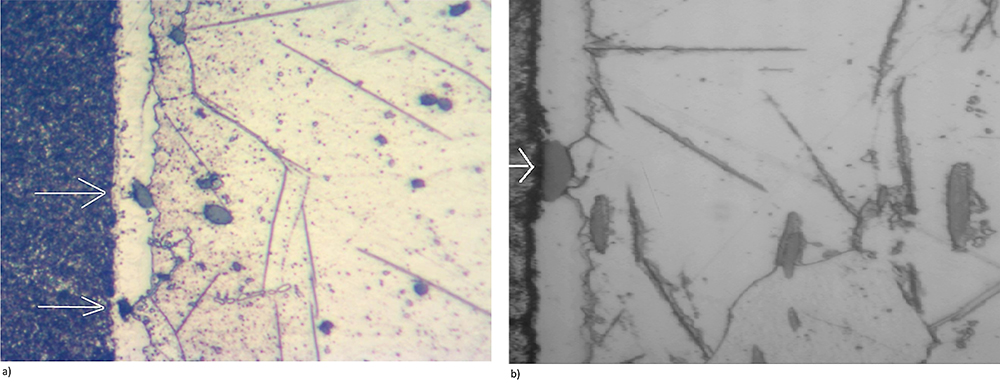
There is a clear negative effect of sulfides on formation of the compound layer and potential for a local imperfection in properties of it. Nevertheless, those inclusions have no practical effect on wear resistance of the steel but may have negative effects on their corrosion resistance.
References
- Thermochemical Surface Engineering of Steels, Ed. E. J. Mittemeijer and M. A. J. Somers, Pub. Woodhead Publishing, 2014.
- T. Blum, M. Chaban, R. Braun, Burgdorf, Uwe Huchel, Heinrich Klümper-Westkamp, Vermeidung von Sperrschichten auf nitrierten Bauteilen – Eine Anleitung zum Handeln, HTM J. Heat Treatm. Mat. 70 (2015) 5 (formerly HTM Z. Werkst. Wärmebeh. Fertigung), pp 248-257. 3
- B. Haase, Surface modification and contamination-Effects on heat treatment, ASM Heat Treating Society, HT Cleaning – 2008.
- G. Wroblewski and K. Skalski, Properties of Surface Layer generated by New Combined Process of Burnishing and Nitriding, Surface Engineering, 2006, Vol. 22, No 2, pp. 138-146.