
Well-designed procedures are crucial to any business operation, whether it’s a simple task of following a procedure to construct a cardboard box, or something more complex such as checking an airplane for safety prior to takeoff. It’s a step-by-step process that guides the person performing the task with written instructions. Its objective is to maintain compliance while minimizing variation, so your company can consistently produce repeatability and desired results for any process that is used either frequently or infrequently. A procedure should be detailed as such that it meets specification and/or standard requirements, yet simple enough that any employee can read through it, follow its instructions to the letter, and achieve the same results.
Some would argue that aligning your company’s procedures in conjunction with specification requirements is easier said than done. Why? Certain areas of your procedures are designed to meet those requirements depending on what sector your business serves, but a major or even slight change will alter how your procedures are written and implemented. Speaking from my own experience, evolving changes had a dizzying effect when locating new requirements and implementing them into our existing procedures. This month’s column will focus on how a gap analysis approach can support the end goal of meeting revision changes, and at the same time minimize the pain of locating and executing those new requirements.
Almost every person in quality knows this situation quite well. A revision or superseding document has been released, and your company uses that document to process and certify jobs. It’s your duty as a quality representative to adapt your processes to the new document. In order to do that you must revise your procedure’s written instructions on how to meet those new requirements. How do you account for all the changes made and align your procedures to meet them?
A system that was introduced to me by one of my mentors, and something I consider to be very effective, is to apply a gap analysis approach. For those of you who are new to this definition or are new to quality in general, gap analysis is a method that measures desired results versus actual results. If your company fails to meet desired results, then there is a gap between desired versus actual. To make applicable to this topic, gap analysis will analyze your existing procedures and find gaps when comparing it to new requirements. The type of gap analysis I use has been called numerous things, but I like to call it a specification accountability sheet.
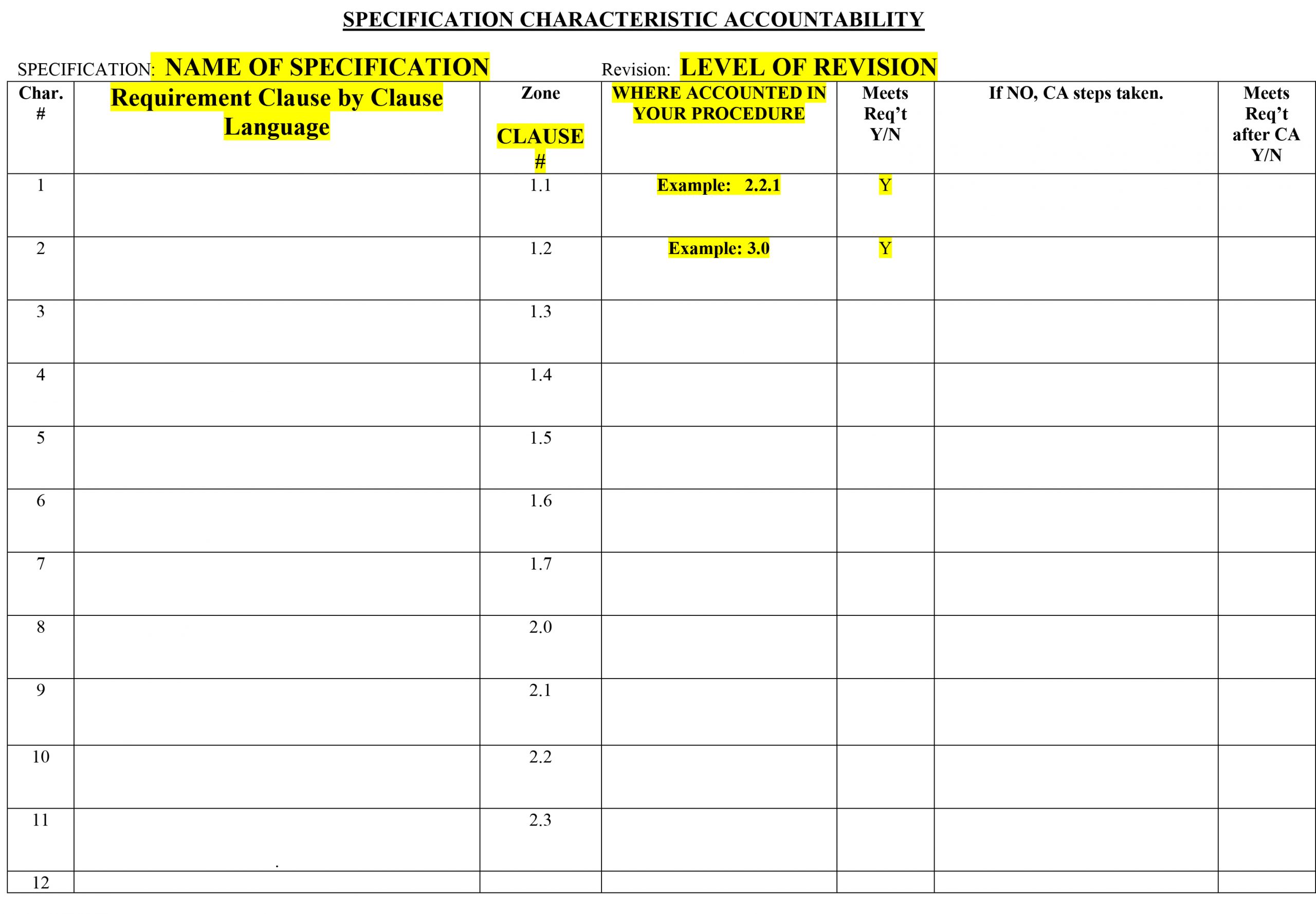 A specification accountability sheet does exactly what its name indicates. It forces you to determine whether your procedures are accountable to a specification. It takes a clause by clause method when determining if your procedures meet the new changes in a document. If your procedure is lacking or not accounting for a new requirement, the sheet will not only show you where your gap lies, but also give you space to make notes and decide on corrective action. The same thing applies when your procedure accounts for new requirements. The sheet will ask you where this clause is accounted for and how it meets the requirement. This method can be monotonous to some, but I find that this is a great tool to dig deep into a revision, provide clear answers on your procedures, and locate where changes need to be made. From my experience when using this method, there are no clauses unidentified, therefore you don’t need to worry about whether you were thorough enough when seeking accountability.
A specification accountability sheet does exactly what its name indicates. It forces you to determine whether your procedures are accountable to a specification. It takes a clause by clause method when determining if your procedures meet the new changes in a document. If your procedure is lacking or not accounting for a new requirement, the sheet will not only show you where your gap lies, but also give you space to make notes and decide on corrective action. The same thing applies when your procedure accounts for new requirements. The sheet will ask you where this clause is accounted for and how it meets the requirement. This method can be monotonous to some, but I find that this is a great tool to dig deep into a revision, provide clear answers on your procedures, and locate where changes need to be made. From my experience when using this method, there are no clauses unidentified, therefore you don’t need to worry about whether you were thorough enough when seeking accountability.
After you’ve accounted for new requirements and made changes to your procedure, it’s time to implement those changes to your staff through training. This is perhaps the easiest thing to do after you’ve spent a considerable amount of time assessing your company’s procedural accountability. It’s also a great opportunity to refresh your staff on the procedure in general. A simple yet straight-to-the-point approach would be to outline each new change and use your own training system to focus on them. There are many ways to do this, so stick with what works best for your company.
Revisions to process specifications are inevitable in the heat-treating industry. New technology or new studies can make discoveries that allow processes to become more efficient, more precise, and achieve superior quality. Although it can be painful to adapt your procedures to these constant changes, being prepared and having a system in place to identify those changes can make the work much easier.
At this point, you should have a good idea on how specification accountability can strengthen your capacity to find gaps within your procedures. It’s a system that requires diligence and persistence from the personnel performing this task, but I can assure you that it will give you peace of mind knowing that you’ve accounted for every change made.























