
In this column, we will discuss the effect of temperature on the cooling curve behavior of quench oils.
Development of Quench Oil Maximum Operating Temperature
The first thing to understand is the operating temperature constraint of the quench oil. This is considered the safe operating temperature of the oil.
The operating parameters of a quench oil are based on the flash point of the oil and the load size. If you consider a typical heat-treating load that follows the “one pound of parts/grids to one gallon of oil,” the oil temperature will increase by approximately 70°F. According to NFPA 86 Standard for Ovens and Furnaces [1], the quench tank should be designed and sized to quench a maximum gross load such that the maximum quenchant temperature is not less than 50°F (28°C) below the flash point of the quenchant. An additional safety factor is added of 50°F (28°C) to allow for hung loads, failed heat exchangers, etc. An additional reason is to minimize oil oxidation. This is shown in Table 1.

This table doesn’t provide any recommendations about the lower limit of the oil, just the recommended upper limit.
Effect of temperature
Increasing the temperature of a quench oil increases the oxidation of a quench oil, so it is a good idea to operate the oil at the lowest possible temperature that will still provide good properties and distortion control.
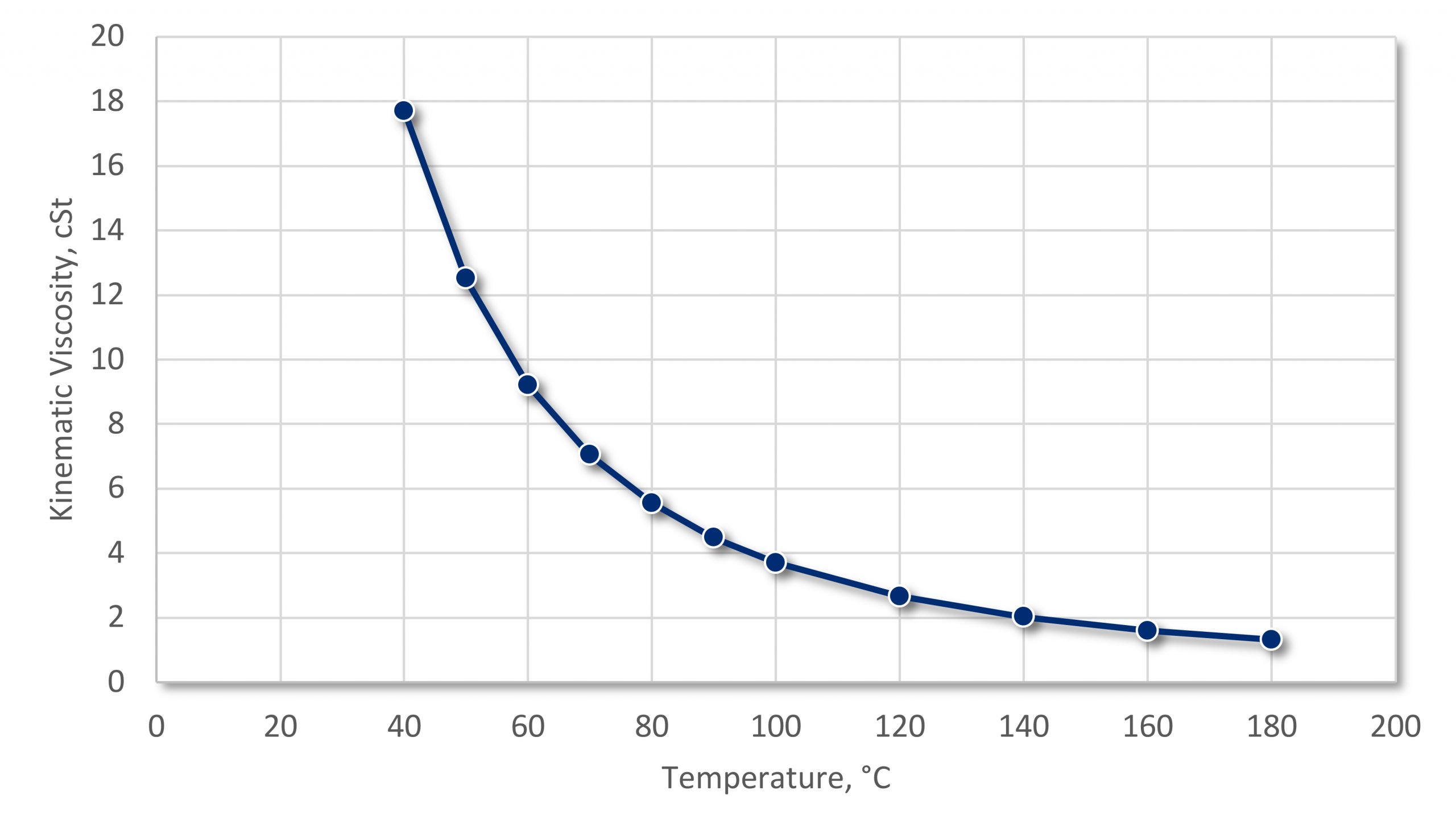
One of the thermophysical properties of a quench oil that changes considerably as a function of temperature is kinematic viscosity. This is the ability to properly wet the part as the part is immersed. If the viscosity is low, then the oil will readily wet the part. If the oil has a high viscosity, then wetting the part becomes more difficult. To visualize this, think of immersing a rod into a vat of water compared to a vat of molasses. Better wetting means that the oil is better able to make intimate contact with the part, and extract heat readily. The change in viscosity as a function of temperature for a medium speed quench oil is shown in Figure 1. The decrease in viscosity as the temperature is increased is typical for all oils.
Using the GM Quenchometer [2], we can look at the overall speed of a quench oil as a function of temperature. Looking at several different oils (Figure 2), we can see that as temperature is increased, the speed of the oil increases (lower number of seconds), reaches a peak, then starts to decrease in speed. Something else is also affecting the heat extraction behavior of the quenchant.
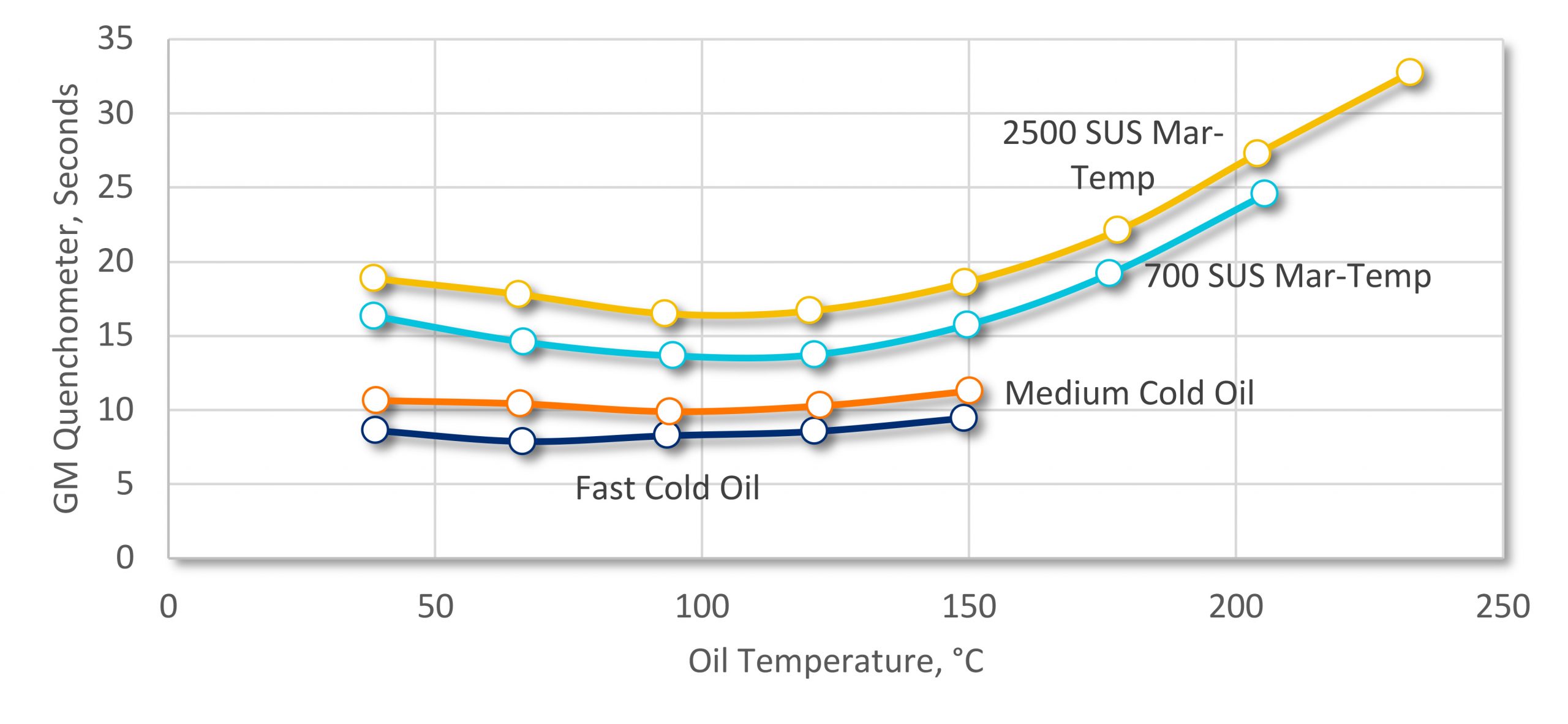
As the temperature of oil increases, the vapor phase during quenching becomes more stable, and longer (more persistent). This stable vapor phase slows down the heat extraction rate. At temperatures below the peak cooling rate, the viscosity effects are dominant. At the peak temperature, neither viscosity nor vapor phase stability effects dominate. Above the peak temperature, the stability of the vapor phase dominates.
As opposed to agitation, different parts of the curve are affected. With agitation, the maximum cooling rates in all three regions are affected and increased. With temperature, only the cooling rates in the vapor phase and nucleate boiling regions are affected. The convection stage remains the same. Further, the effects of temperature are more subtle, without the large effects of agitation. The effect of temperature on a thick mar-tempering oil with a nominal kinematic viscosity of 550 cSt at 40°C is shown in Figure 3.
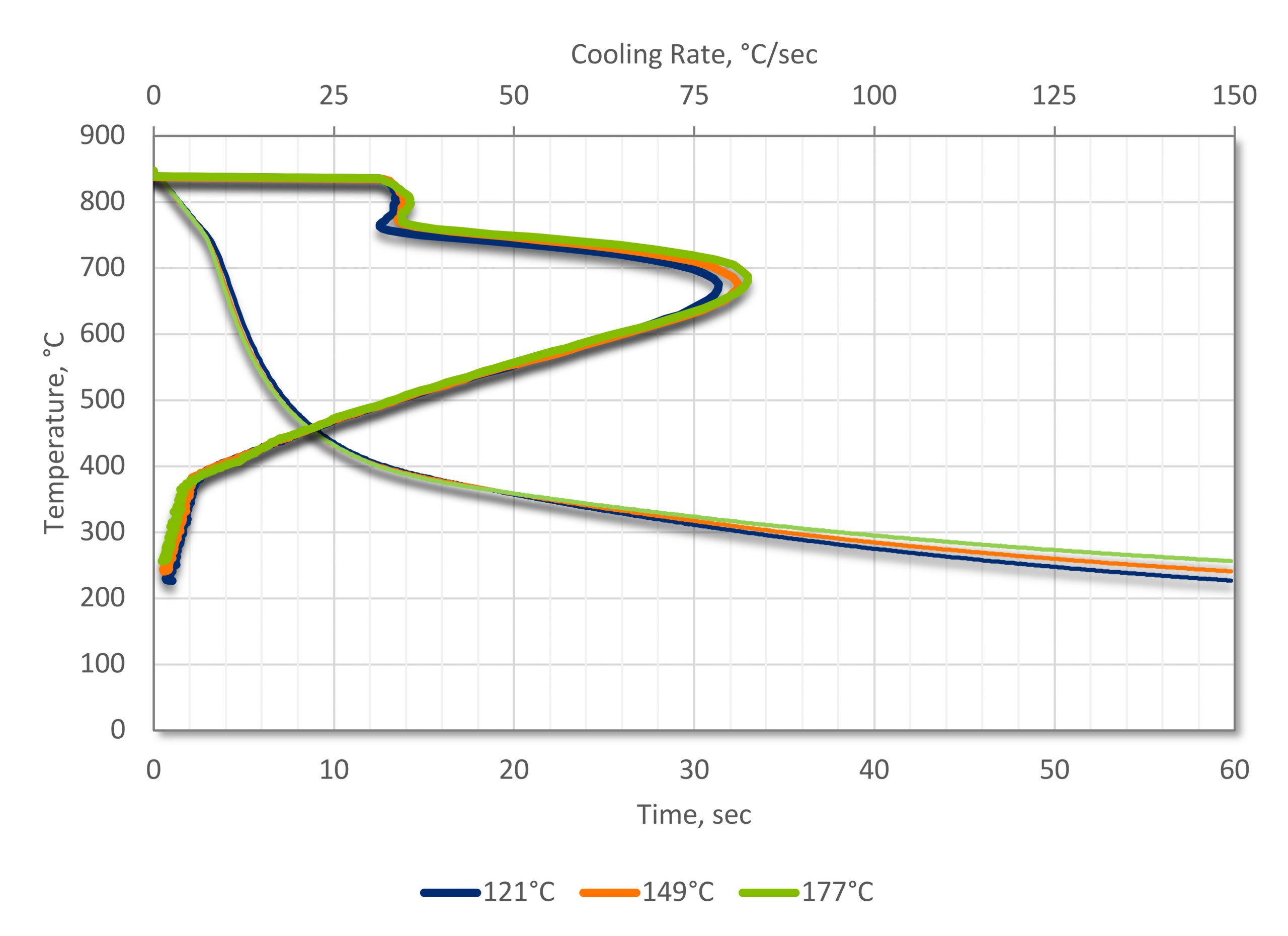
The benefits of increasing quenchant temperature are a slight increase in the overall quenching rate. The biggest advantage of increasing the quenchant temperature is reducing the thermal gradients in the part during quenching. This reduction of thermal gradients reduces the residual stresses formed in the part during quenching and reduces distortion. This is the primary purpose of mar-tempering oils. However, some reduction in distortion can also occur in cold oils by increasing the operating temperature to the maximum operating temperature.
Conclusions
In this article, the effect of quenchant temperature on the cooling curve behavior was shown. Increasing the temperature of the quenchant can offer better wetting of the part and increased heat extraction. This can help with achieving properties. Increasing the temperature of the quenchant can also help reduce the residual stresses and the amount of distortion occurring in a part. The effect of temperature, working in conjunction with agitation, can yield parts with excellent properties and reduced distortion.
Should you have any questions regarding this column, or suggestions for future articles, please contact the author or the editor.
References
- National Fire Protection Association, “Standard for Ovens and Furnaces,” Quincy, MA, 2015.
- ASTM, “Standard Method for Quenching Time of Heat-Treating Fluids (Magnetic Quenchometer Method),” American Society for Standards and Materials, Conshocken, PA, 88.

























