
Modern concerns for sustainable manufacturing are driving significant interest and investment in hydrogen as an industrial fuel. Because burning hydrogen produces no carbon emissions, it provides a promising option for industry to reduce carbon emissions. Burning hydrogen for industrial applications has been done for over a century, but only in selected industries with a ready source of hydrogen.
The science of burning hydrogen is well known, but converting to the fuel does not come without some risks and design considerations. This article will explain hydrogen basics and explore some concerns to be aware of in using the fuel.
Hydrogen as a fuel
When hydrogen is produced using a renewable energy source, it results in being a CO2 neutral form of fuel.
When natural gas is burned (assuming for simplicity that it is 100% CH4) you produce CO2, water vapor, and energy.
![]() When you burn 100% hydrogen, there is only water vapor and energy that results.
When you burn 100% hydrogen, there is only water vapor and energy that results.
![]() So, hydrogen as a fuel source is attractive in being able to reduce or eliminate the carbon produced by thermal processes.
So, hydrogen as a fuel source is attractive in being able to reduce or eliminate the carbon produced by thermal processes.
Hydrogen Combustion Characteristics
- The H atom is the lightest and smallest element; it is eight times lighter than natural gas.
- It is colorless, odorless, and tasteless.
- The H2 flame burns about eight times as fast as natural gas and with a higher flame temperature (approximately 400°F hotter than natural gas)
- H2 is extremely flammable with a very wide flammability range in air (LEL to UEL (vol%) of 4-77) compared to natural gas at (5-15%).
- 15 times lower spark energy is needed to ignite H2 than natural gas.
- The heating value by volume is 3-3.5 times less than commercially available natural gases.
Burner and System Considerations
Hydrogen looks to be very suited as a fuel in combustion processes being more flammable and requiring less energy to ignite. However, burner designs vary widely to accommodate process requirements such as flame shape/velocity, emissions, efficiency, turndown, etc. So, what are some of the critical factors when considering H2? For those, we look at burners and their systems:
Materials of construction: Burner materials of construction will need to be examined and often upgraded to accommodate increasing flame temperatures with increasing percentages of H2 in natural gas up to 100%.
Burner design and selection: The method of air/fuel mixing design often will dictate how an existing burner design will react to the introduction of H2 as the fuel source. Some burner designs require little or no change to be able to burn up to 100% H2; however, burner designs using partial or full premix and/or high swirl are challenging, if not impossible, with the high flame speed of H2.
If any degree of H2 premix is intended with a burner application, be aware the very high flammability nature of hydrogen premix may require the use of additional equipment such as flashback arrestors or blow outs similar to high pressure natural gas premix.
Overall, do not assume H2 can be used in any burner. Due to the nature of the fuel, always consult with equipment suppliers to determine if hydrogen fuel in a burner for your application is recommended and what, if any, changes are required.
Flame detection: UV sensors have been applied with H2/gas mixtures up to 100% H2 with no issue sensing the flame. Flame rod (ionization), flame rectification will become weaker as the percentage of H2 in the gas becomes higher. Above 80-90% H2 in the gas mixture, UV flame detection is recommended.
Gas pressure: To maintain the same thermal input, a burner will need higher gas pressure burning H2 vs. natural gas since the heating value of H2 by volume is about a third of natural gas. This pressure of course varies if you are burning H2/Natural Gas blends.
Stoichiometric air required for combustion: General rule of thumb for natural gas combustion is a stoichiometric air/fuel ratio of about 10:1, so 10 cubic feet of air is required for every one cubic foot of natural gas burned. The approximate stoichiometric air/fuel ratio for hydrogen is 2.4:1, so 2.4 cubic feet of air is required for every one cubic foot of hydrogen burned. If we need 3.5 times the volume H2 to equal the given heat input using natural gas, then for equal heat release we would need 3.5ft3 H2 ∗ 2.4 ft3 air/ft3 H2 = 8.4ft3 air for hydrogen. Or we need about 85% of the air volume for pure H2 combustion versus natural gas combustion.
If you intend to fire varying percentages of natural gas/H2 blends, or at times 100% of either fuel, then depending on your application, you may have to look at your burner control system and ability to adapt air fuel ratio (excess air), if that is important in your process or for your product.
Fuel Safety trains and control valves: When you consider blending or running your system on a blend or up to 100% H2, then the safety and control equipment and materials of construction that are part of that system need to be suitable for H2 service. The majority of our process heating systems runs at low fuel temperatures and pressures, so hydrogen embrittlement should not be a concern. For your safety and control devices, you should confirm their suitability for hydrogen service.
Due to the very low heating value of hydrogen, a much higher volume of the fuel must be fed to a burner for a similar total heat input. As such, fuel handling components like regulators, control valves, mixers, and shut off valves may have to be replaced or added to allow the larger volume of fuel to be fed to burner at a given supply pressure.
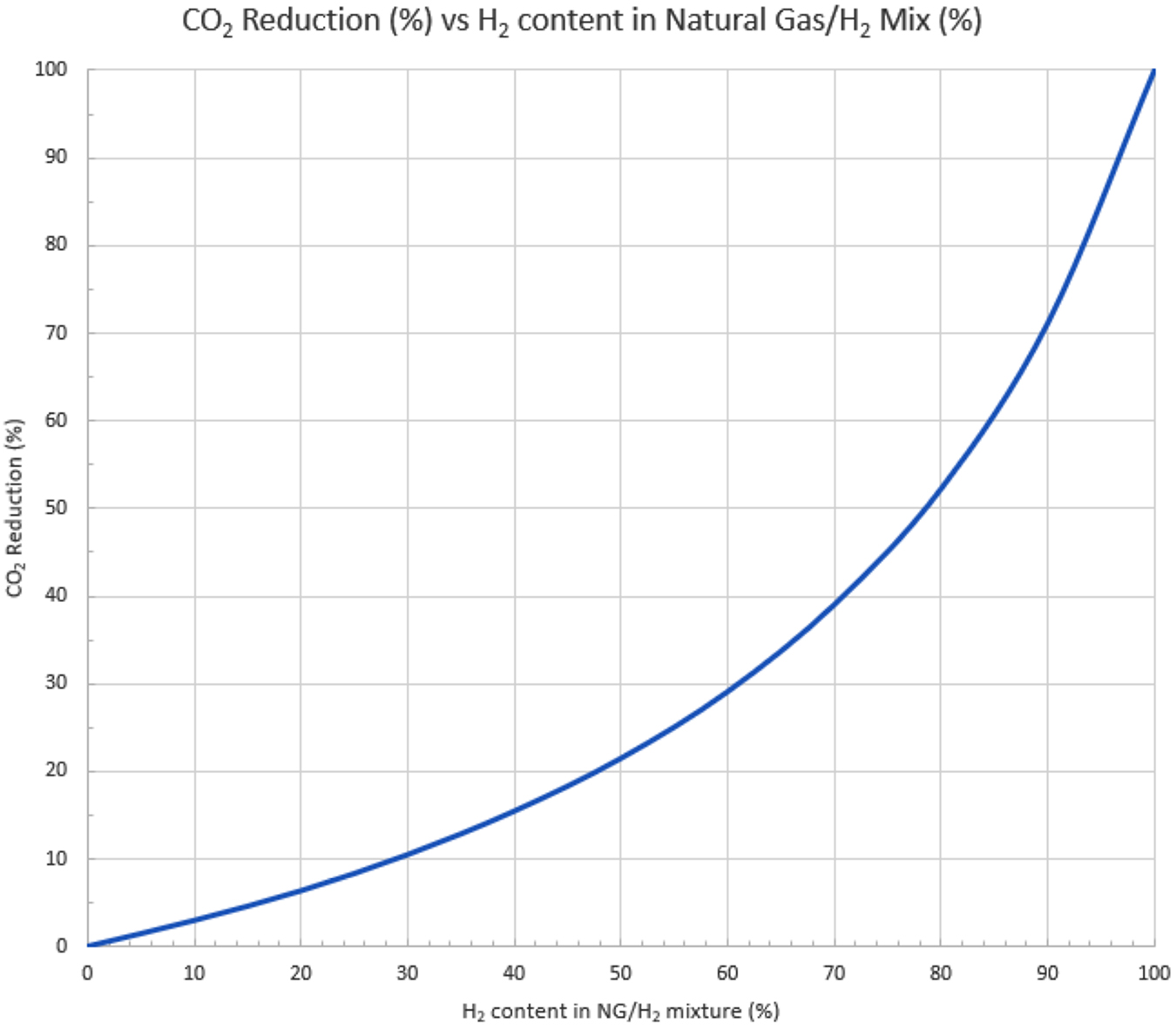
CO2 reduction with natural gas/H2 blending: If you consider the blending of hydrogen with natural gas to start reducing CO2 emission, be aware that the percentage of hydrogen in the mixture is not a direct correlation to the amount of CO2 reduction in the combustion products. This is a result of hydrogen’s lower heating value and very low density. You can see in the Figure 1 that to realize a 50% reduction in CO2 emissions, you need almost 80% hydrogen in your hydrogen/natural gas blend.
Emissions: The impact of H2 on NOx will be highly influenced by the burner design. We have seen increases in volumetric (ppm) NOx levels as the percentage of H2 increases in the gas mixture resulting from the increase in flame temperature. But this volumetric reference/measurement can be misleading because of the change in volume of products of combustion and other corrections when firing H2. So volumetric comparison (e.g., ppm) should be avoided and a mass/unit energy is recommended to compare NOx from natural gas vs H2 combustion.
Examples
NOx (Natural Gas)
50ppm (corr. to 3% O2) = 0.060 lbs/MM Btu
NOx (H2)
50ppm (corr. to 3% O2) = 0.041 lbs/MM Btu
So, to equal the same lbs/MM Btu with H2 as with natural gas the conversion is:
0.060 lbs/MM Btu = 73ppm (corr. to 3% O2)
So be aware of this when environmental agencies and regulations are of concern.






















