
Intergranular oxidation (IGO) in carburizing steels is detrimental to carburized gear performance. It reduces fatigue performance and can contribute to surface spalling. This article discusses the causes of intergranular oxidation and methods to reduce it.
Introduction
Intergranular oxidation during carburizing has been known for at least 70 years [1]. Intergranular oxidation is caused by the precipitation of oxides of one or more of the alloying elements on grain boundaries. Alloying elements such as silicon (Si), chromium (Cr), and manganese (Mn) have a greater affinity for oxygen than iron. The presence of these internal defects strongly impacts fatigue properties and can severely shorten the expected life of carburized steels used in drive train or other loaded applications.
Near the surface, complex oxides of manganese and chromium dominate [2]. These oxides form as globules within grain boundaries, or within the surface grain. A short distance from the surface, oxides of silicon form a grain boundary network (Figure 1). These internal and surface oxides can act as initiation sites for fatigue, reducing expected life.
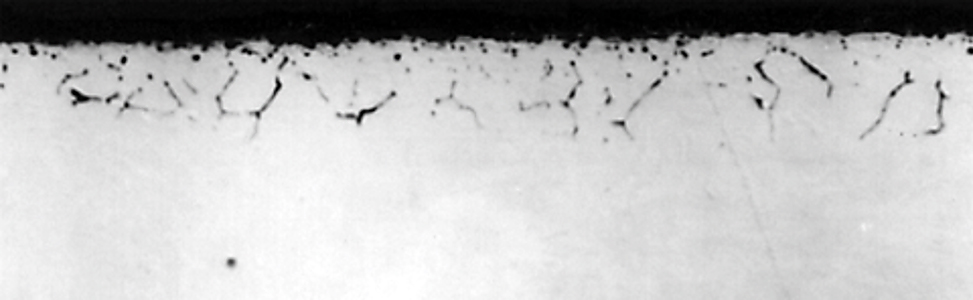
During the formation of internal oxides, the matrix is depleted of alloying elements. This reduces the hardenability of the alloy at the surface and near the surface [1]. Because of the reduced hardenability, there may be non-martensitic transformation products such as pearlite, ferrite, or bainite formed, instead of the expected martensite. Due to the lower hardenability, increased quench rates are required to prevent the formation of non-martensitic transformation products. However, this can also lead to increased distortion. Increasing molybdenum (Mo) and nickel (Ni) content will increase the hardenability of the steel and reduce the depth of the non-martensitic transformation product depth [5].
Effect of Alloying Elements
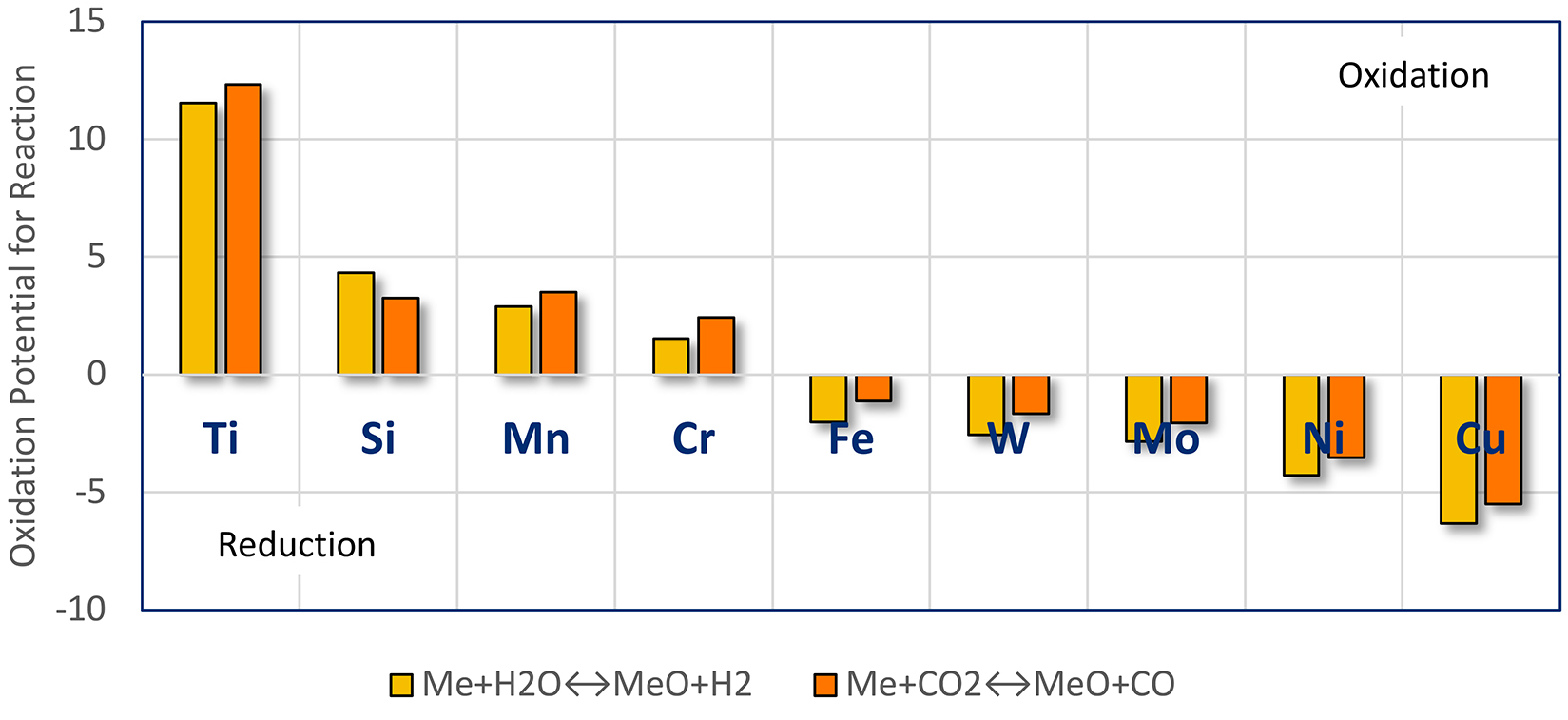
The partial pressure of oxygen in a carburizing atmosphere is on the order of 10-20 atm [6]. This partial pressure is reducing with respect to iron, but other oxides have much lower equilibrium partial pressures. The oxides of Si, Mn, and Cr have equilibrium oxygen partial pressures of 10-24 to 10-30 atm. This means that these alloying elements can be selectively oxidized during carburizing. This oxidation usually occurs along grain boundaries because this is a low-energy path for diffusion; however, internal oxidation within grains can and does occur. Additional oxidation can occur during loading or unloading.
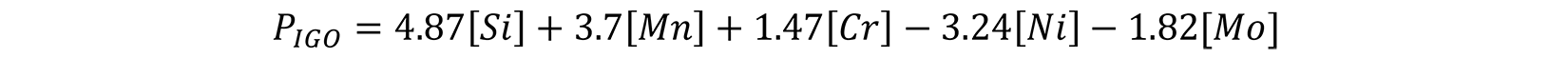 Another study [9] found that internal oxidation increased with increasing Si, Mn, and Cr content, until it reached a limiting value. Further increases in alloy content decreased the amount of IGO. The limiting value was found to be 0.5-0.6% Si, 0.8% for Cr, and Mn about 2.1%.
Another study [9] found that internal oxidation increased with increasing Si, Mn, and Cr content, until it reached a limiting value. Further increases in alloy content decreased the amount of IGO. The limiting value was found to be 0.5-0.6% Si, 0.8% for Cr, and Mn about 2.1%.
Carburizing Practice
To minimize intergranular oxidation, it is necessary to minimize the amount of oxygen present. Chatterjee-Fischer [10] found that by increasing the carbon potential, reducing the CO2 content, IGO was reduced. This was thought to be related to carbon reacting with O2, reducing the free oxygen potential. For a 1% Cr steel, internal oxidation was minimized when the CO2 content was no more than 0.2% maximum, while for a 1% Mn steel, the maximum CO2 content was 0.1% [11]. Edenhofer [12] found that when doubling the CO content from a nominal 20% to 40%, the amount of IGO was doubled.
The time during carburizing has been found to increase with the square-root of time [10]. Increasing temperature increases the amount of intergranular oxidation.
When using nitrogen-methanol atmospheres, the purity of nitrogen matters. In-situ generated nitrogen contains greater amounts of oxygen than does cryogenically generated nitrogen. This means that more IGO will be created with in-situ generated nitrogen than cryogenic nitrogen. Direct injection of natural gas (CH4) will help scavenge oxygen and reduce IGO.
 Conclusions
Conclusions
In this article, silicon is the most significant alloying element causing intergranular oxidation. It has the lowest free energy of formation. Based on atomic size, Si is much smaller than Cr or Mn, and so has the highest diffusion rate. Higher carbon potential tends to reduce IGO, by carbon reacting with free oxygen. The formation of IGO tends to reduce the local hardenability of the steel, contributing to non-martensitic transformation products at the surface. Only fast quench rates, capable of overcoming the lowered hardenability, will reduce the formation of non-martensitic transformation products.
Should you have any questions regarding this column, please contact the writer or editor. Suggestions for new columns are very much welcomed.
References
- G. Parrish, “Carburizing: Microstructure and Properties,” Metals Park, OH: ASM International, 1999, pp. 11-36.
- X. An, “PhD Dissertation: A Study of Internal Oxidation in Carburising Steels,” Sheffield, UK, 2002.
- D. Matlock, K. A. Alogab, M. D. Richards and J. G. Speer, “Surface Processing to Improve the Fatigue Resistance of Advanced Bar Steels for Automotive Applications,” Materials Research, vol. 8, no. 4, pp. 453-459, 2005.
- R. Gorockiewicz, A. Adamek and M. Korecki, “The benefits of using 3 Gas Mixture Low Pressure Carburizing (LPC) for high alloy steels,” in Proc. of the 24th ASM Heat Treating Conf. 17-19 September 2007, Detroit. MI, 2007.
- W. E. Dowling, W. T. Donlon, W. B. Copple and C. V. Darragh, in Carburizing and Nitriding With Atmospheres: Proceedings of the Second International Conference on Carburizing and Nitriding With Atmospheres 6-8 December 1995, Cleveland, OH, 1995.
- Linde Group AG, “Furnace Atmospheres #1: Gas Carburizing and Carbonitriding,” Linde AG, Unterschleissheim, Germany, 2000.
- I. S. Kozlovskii, A. T. Kalinin, A. Y. Novikova, E. A. Lebedeva and A. I. Feofanova, Metal Sci. Heat Treat., vol. 3, pp. 157-161, 1967.
- M. Lohrmann and W. Lerche, “Investigation of influences by the process and the steel on the extent of internal oxidation during gas carburizing,” Harterei-Technische Mitteilungen, vol. 55, no. 6, pp. 372-380, 2000.
- N. Murai, K. Aihara, T. Tsumura and K. Nishida, Sumitomo Metals (Japan), vol. 45, no. 4, pp. 11-21, 1993.
- R. Chatterjee-Fischer, “lnternal Oxidation During Carburizing and Heat Treating,” Met. Trans A, vol. 9A, no. 11, pp. 1553-1560, 1978.
- C. Dawes and R. J. Cooksey, “Special Report 95: Heat Treatment of Metals,” Iron and Steel Insitute, London, UK, 1966.
- B. Edenhofer, in Proc. of the Second International Conference on Carburising and Nitriding with Atmospheres, Cleveland, OH, 1995.






















