
In the last article, we talked about the metallurgy behind quenching aluminum. Now we are going to discuss the available quenchants for aluminum.
Introduction
To achieve proper quenching of aluminum, the solute must be maintained in solid solution. This means that quenching must be rapid to prevent premature precipitation of solute. If this occurs, the solute will precipitate at grain boundaries, and be lost for any further hardening [1].
For aluminum heat-treating, quenchants are usually water (hot or cold, depending on the alloy and product form), or polyalkylene glycol (PAG).
Water
Water quenching is the most readily available and most common quenchant for wrought and cast aluminum. Typically, quenching aluminum in water is conducted at either room or elevated temperatures (20-80°C). Water quenching has many advantages, including being readily available and inexpensive. There are no storage problems or health and safety problems associated with storage or disposal of water. It is nonflammable and nontoxic, and has no smoke or fumes. Cleaning is not required prior to subsequent processing. It is easy to pump and filter and is readily compatible with many different metals and polymers.
However, water quenching is not a panacea, and has disadvantages that restrict its use to specific applications. Often, if materials for the quench tank are not chosen properly, corrosion of the quench tank, fixtures, and fittings can occur. In addition, water supports biological growth. Corrosion inhibitors and biocide packages can be used to overcome corrosion and bacteria growth. However, the quenching characteristics of water can cause problems with distortion and cracking. Water also shows significant variation in quenching characteristics depending on the hardness of the water. Hard water is much faster than distilled water (Figure 1), which can result is property and distortion differences.
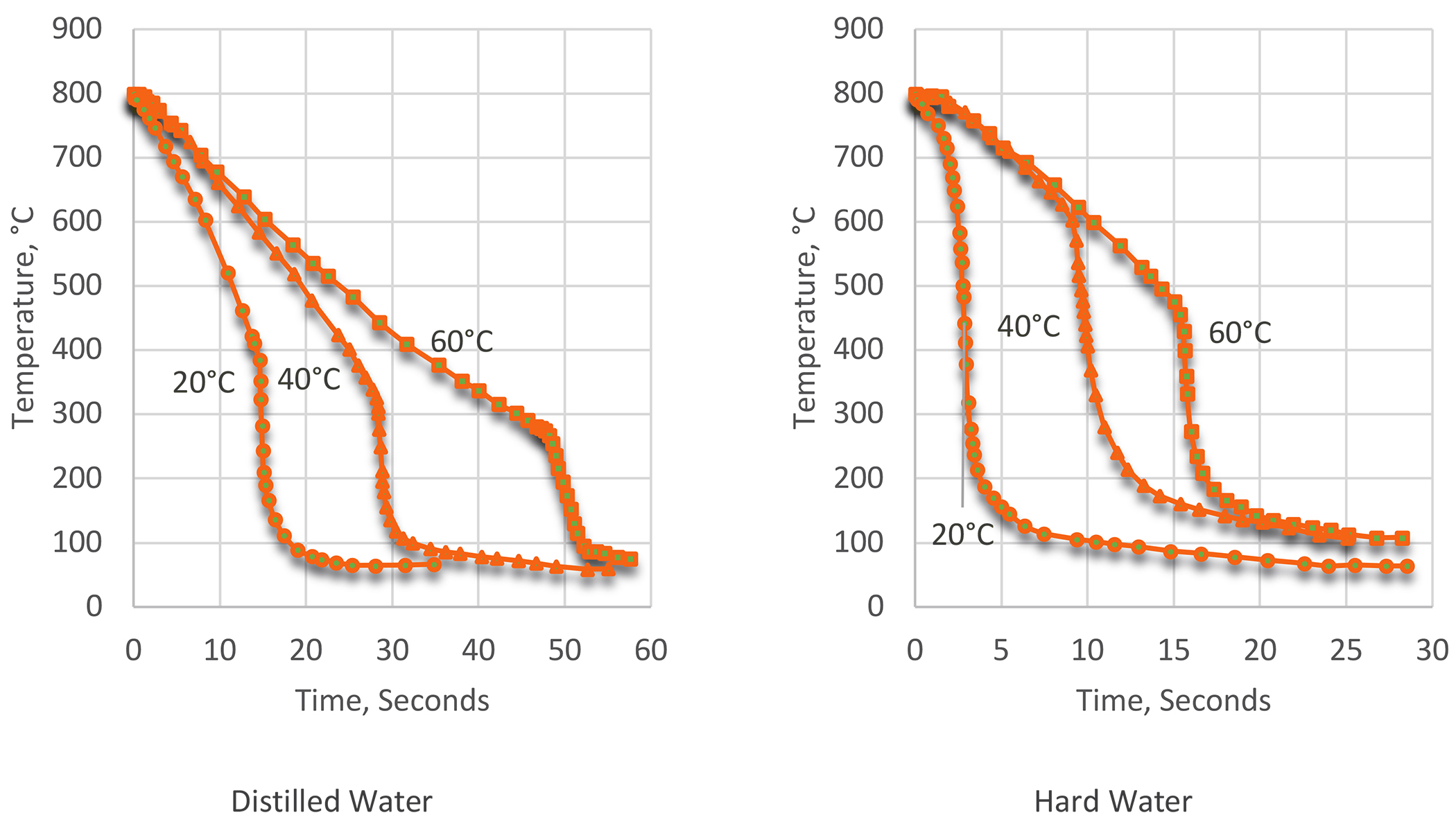
The cooling rate of water quenching is independent of material properties such as thermal conductivity and specific heat. It is primarily dependent on water temperature and agitation [2]. Water temperature is the largest primary variable controlling the cooling rate. With increasing water temperature, the cooling rate decreases. The maximum cooling rate also decreases as the water temperature is increased. In addition, the temperature of maximum cooling decreases with increasing water quench temperature. The length of time and stability of the vapor barrier increases, with increasing water temperature. This is shown in Table 1.
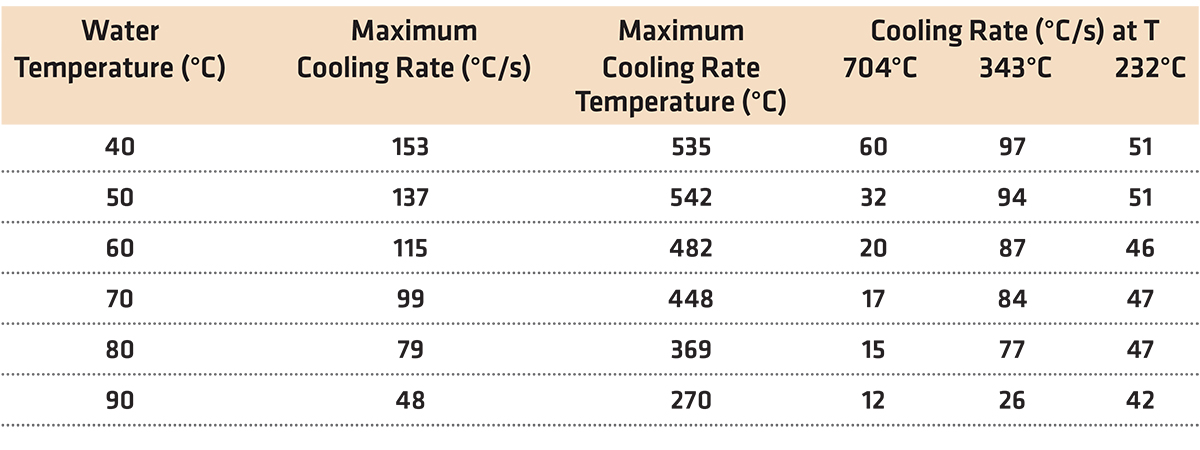
As water temperature increases, the vapor phase becomes prolonged, and the maximum rate of cooling decreases sharply. This can lead to soft spots, high conductivity, or locations of intergranular corrosion susceptibility in the workpiece.
The stability of the vapor phase is dependent on the surface finish of the component. The vapor film is extremely persistent on flat or smooth surfaces. The vapor film is easily trapped on bottom surfaces, and in small pockets. Extremely rough surfaces can cause the retention of the vapor phase, and limit heat transfer. Adjacent parts can also cause film retention. This film breaks up readily with the onset of boiling at sharp corners, rough surfaces, defects, or other stress risers. This variation in stability will cause different cooling rates across the part, resulting in distortion and cracking.
Water also exhibits extremely high cooling rates in the convection phase. High cooling rates can help make sure that the part meets mechanical properties but can also cause excessive residual stresses or part cracking to form. The detrimental effects of temperature dependence and vapor phase stability can be minimized by maintaining the water at a low temperature through effective cooling, and vigorous agitation to disperse the vapor phase.
Cold water quenching is the most severe. In an early study using cooling curves [3], it showed that quenching into still water caused rapid heat transfer. This study showed that heat transfer at the surface of the part was very turbulent at the metal/water interface. This study also showed that there was a marked difference between hard water and distilled water. Distilled water showed an extensive vapor blanket that extended to low temperatures.
Quenching into water at < 50-60°C often produces non-uniform quenching. This non-uniformity manifests itself as spotty hardness, distortion, and cracking. This non-uniformity is caused by relatively unstable vapor blanket formation. Because of this difficulty, it was necessary to develop an alternative to water quenching. Polyalkylene glycol quenchants (PAG) [5] were developed to provide a quench rate in between that of water and oil. By control of agitation, temperature, and concentration, quench rates like water can be achieved.
The average cooling rates of quenching aluminum in water have been measured for different plate thicknesses (Figure 2). A wide variation in average quench rates are observed when the temperature of the water is changed. It is seen that the average quench rate at 400-290°C decreases as the water temperature is increased.
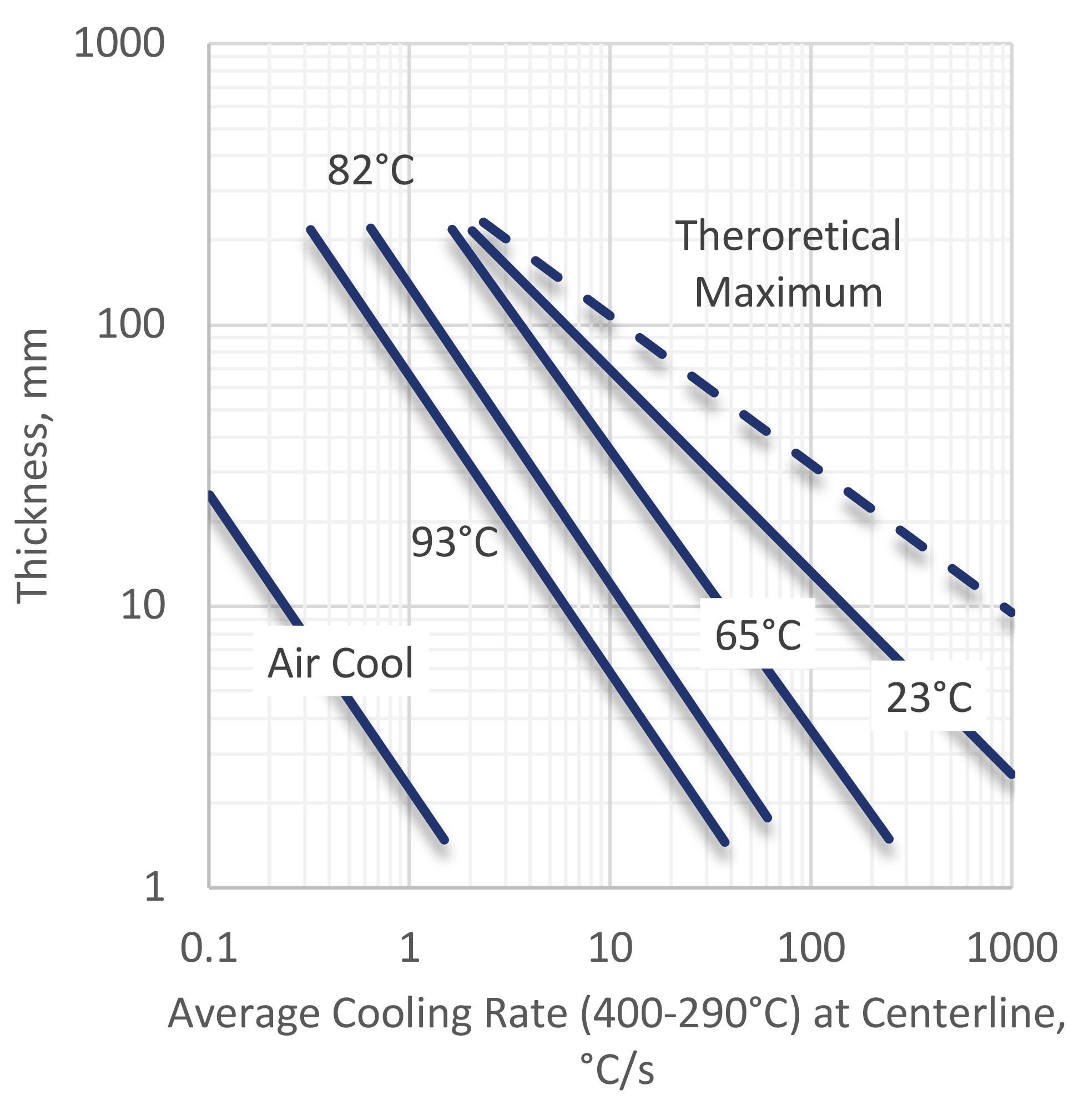
For very thick plates, it is not possible to obtain full properties because the cooling rate is not adequate to keep the solute in super-saturated solid solution. Some precipitation of the solute at grain boundaries will occur. This must be considered during design.
Conclusions
In this short article, we have discussed the use of water for quenching aluminum. In the next article, we will discuss the application of polyalkylene glycol quenchants for quenching aluminum.
Should there be any questions regarding this article, or any suggestions for further articles, please contact the author or editor.
References
- J. E. Hatch, Aluminum: Properties and Physical Metallurgy, Metals Park, OH: American Society for Metals, 1984.
- A. Rose, Arch. Eisenhulles, vol. 13, p. 345, 1940.
- K. Speith and H. Lange, Mitt. Kaiser Wilhelm Inst. Eisenforssch, vol. 17, p. 175, 1935.
- G. Totten, C. Bates and N. Clinton, Eds., Handbook of Quenching and Quenchants, Metals Park, OH: ASM International, 1993.
- SAE, “AMS 3025E Polyalkalene Glycol Heat Treat Quenchant,” SAE, Warren, PA, 2018.





















