In this column, I will discuss the inorganic salt quenchants and how they can be used effectively to quench lean hardenability steel parts.
Often, a heat treater is presented with a situation that neither oil, water, nor polymer can solve. Situations such as having a very lean alloy, with a very high hardness requirement, that requires a faster quenchant than water, oil, or polymer quenchant can provide. The use of aqueous salt quenchants can provide the answer in this situation.
Aqueous inorganic salt quenchants are quenchants that use small amounts of inorganic salts to achieve faster and more uniform quenching compared to water. Even small amounts of dissolved salt in water can drastically change the quenching characteristics. These changes due to the presence of inorganic salts can overcome many of the disadvantages of water. These types of quenchants are often referred to generically as “brine” quenchants, the term being coined due to the use of salt water in older quench tanks.
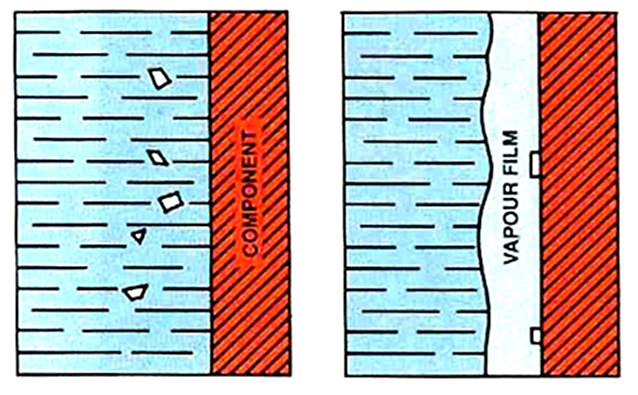
Salts, acids, and alkalis readily dissolve in water. These act to reduce the stability of the vapor phase during quenching. If the concentration is high, then the vapor phase will not form at all. This type of contamination can be taken advantage of to create quenchants with very fast quench rates.
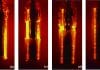
During quenching, minute crystals of salt precipitate on the surface of the part (Figure 1). This causes water to be displaced and the waters of hydration to escape. The localized high temperatures cause these crystals to fragment violently, and this creates turbulence, which destroys the vapor film and initiates nucleate boiling [1]. This yields very high maximum cooling rates.

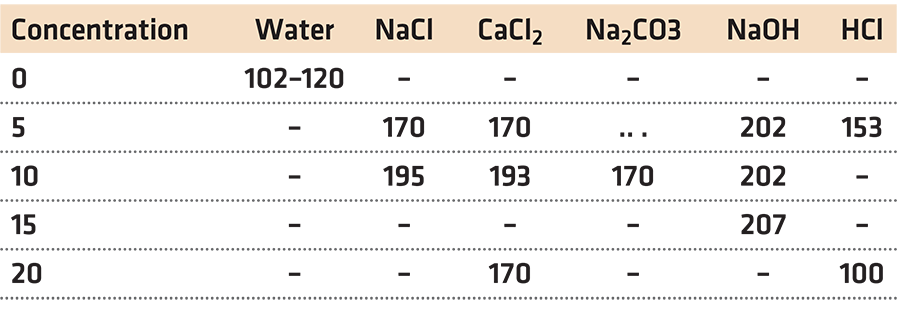
The most common aqueous inorganic salt quenchant is a solution of sodium chloride and water. As the concentration of sodium chloride increases, the faster the cooling curve. This in turn, increases the hardness of quenched steel (Figure 2). The vapor phase disappears as the concentration is increased to 10 percent or greater.

Other salts behave in a similar manner to sodium chloride. Alkali solutions such as NaOH show a similar response (Table 1).
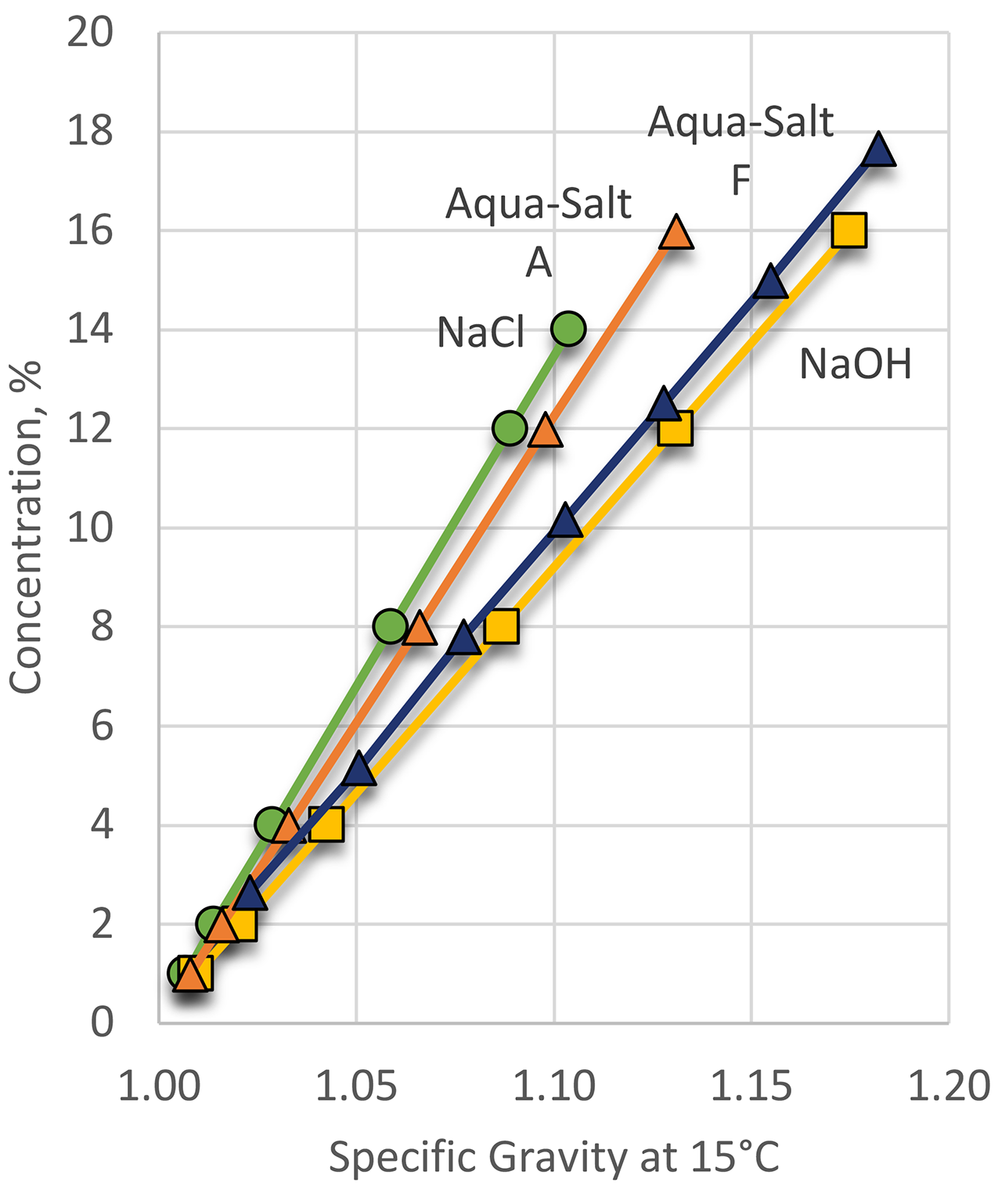
While the use of sodium hydroxide or sodium chloride is effective, it is also hazardous. Sodium hydroxide (lye) and sodium chloride are very corrosive. To answer that need, proprietary salt mixtures such as Aqua-Salt™ A and Aqua-Salt™ F were developed to overcome the deficiencies of traditional salt of alkali quenching and provide more efficient quenching (Figure 3).
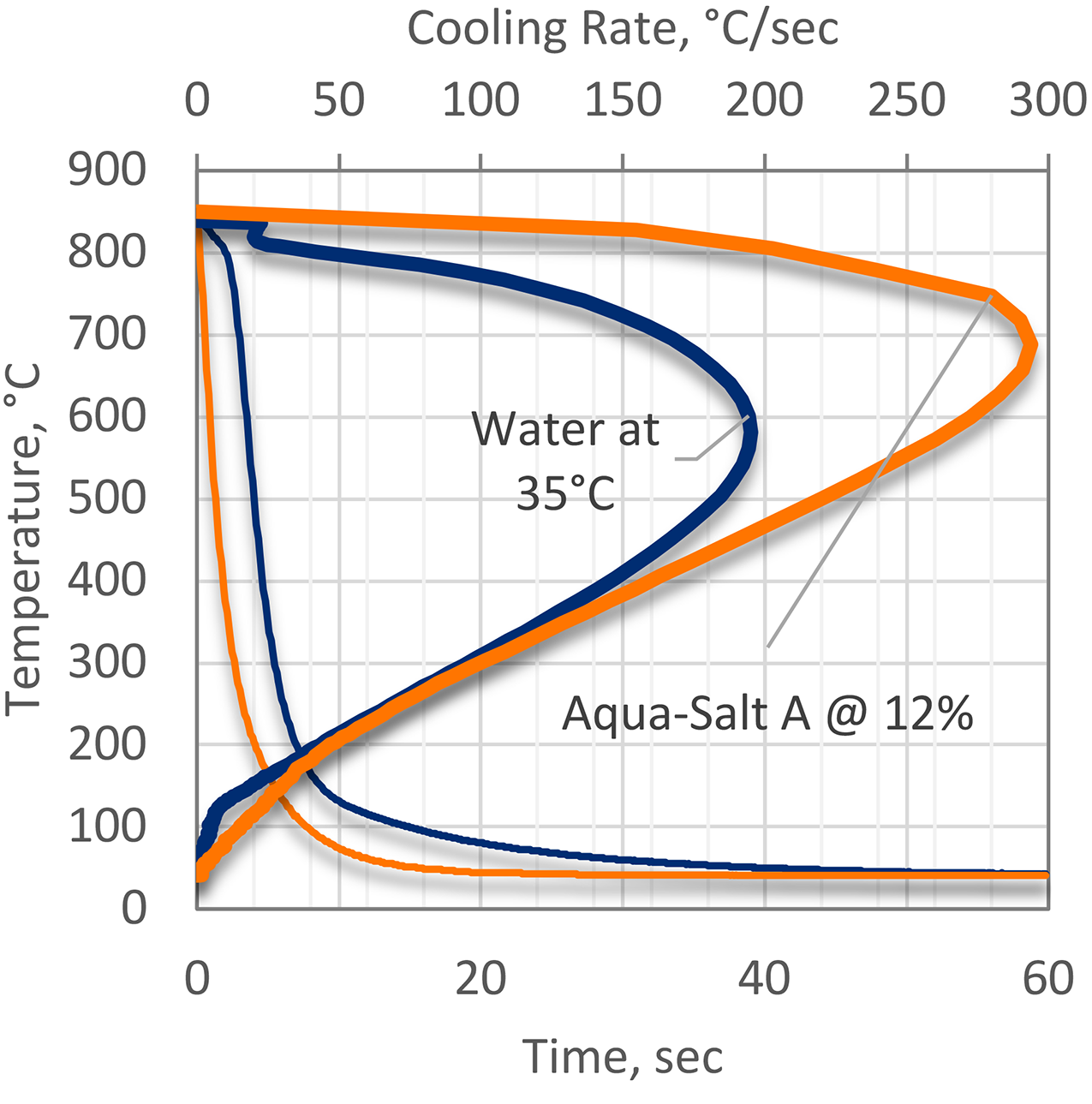
The quenching power of aqueous inorganic salt solutions is not affected by temperature to the same extent that water is. While the temperature range of inorganic salt solutions is wide, the optimal temperature is approximately 20–40°C.
The concentration of aqueous inorganic salt solutions is expressed usually by specific gravity. The concentration of aqueous salts can be determined in several ways; however, the simplest method is using a simple hydrometer measuring the specific gravity of the solution (Figure 4).
A hydrometer is a simple instrument that operates using the Archimedes principle. That is, a solid body will displace its own weight within a liquid. There are two types of scales — one for liquids lighter than water and another for liquids heavier than water. For the case of aqueous salt solutions, the scale for liquids heavier than water is used. In other words, for a specific gravity greater than 1.000.
A hydrometer is usually made from a tall glass stem that is weighted at one end to make it float upright. The liquid to be tested is placed into a tall container, such as a graduated cylinder. The hydrometer is gradually lowered into the fluid. There are usually graduations along the side of the hydrometer. The hydrometer floats in the fluid, and where the liquid surface touches the stem of the hydrometer is noted. Usually, the specific gravity of the fluid can be read directly from the hydrometer.
The accuracy of the hydrometer depends on three main factors: the cleanliness of the hydrometer, the temperature of the fluid, and ensuring proper immersion.
The cleanliness of the hydrometer is very important. It should be properly cleaned after each use so that the fluid will properly wet the surface.
The hydrometer is calibrated for a certain temperature (usually 15.5°C). The fluid should be at this temperature for accurate readings.
Lastly, the graduated cylinder, or other container, should have a diameter approximately 2.5 cm greater than the diameter of the graduated cylinder height to minimize meniscus effects.
The major advantages of aqueous salt solutions for quenching are:
• Aqueous salt solutions are relatively not affected by bath temperature to the same degree that water is.
• The vapor phase is eliminated. Quenching is more uniform, even in dense loads.
• Uniform quenching is more readily achieved. Soft spots and distortion are not usually an issue, provided that proper low alloy steels are selected.
• The quench rate is substantially greater than water. As-quenched and hardness uniformity is increased.
The disadvantage of aqueous salt solutions as quenchants are:
• The quench tank and the support equipment (hoists, racks, agitators, etc.) must be protected from corrosion. Quench tanks are most often constructed of stainless steel. More attention must be paid to proper grounding to prevent stray currents, and material compatibility. Increased use of rust inhibitors is required, unless quenchants are chosen that incorporate the necessary corrosion inhibitors.
• Hoods and exhaust stacks are necessary to remove aqueous salt vapors. Stacks and exhausts should be made of stainless steel to prevent excessive deterioration of the ductwork.
• The material and labor costs associated with brine quenchants are higher. This is because of the costs of concentration control, preparation of solution and raw material costs. Maintenance costs are also higher because of corrosion.
• Environmental costs are also higher, as the cost of disposal is increased.
Many of these issues can be overcome with proper engineering controls.
Conclusions
In this article, the mechanism of quenching for aqueous salt or brine quenchants is described. Precipitation of the salt initially forms on the surface of the part, defeating the vapor phase, starting nucleate boiling early. This results in a very fast quench.
Aqueous salt quenchant concentration is commonly determined by using a common hydrometer to measure specific gravity of the solution. The concentration of the salt is linearly dependent on the specific gravity of the solution, so concentration can be simply determined.
The biggest benefits of aqueous salts are their very fast quench rates and relative immunity to agitation and temperature effects. This simplifies quench tank design.
The primary disadvantage is corrosion. However, proper design can mitigate many of the corrosion issues.
References
- L. H. Zordao, V. A. Oliverira, G. E. Totten and L. Canale, “Quenching power of aqueous salt solution,” Int. J. Heat Mass Transfer, vol. 140, pp. 807-818, 2019.
- D. S. MacKenzie, “Quenchants Used for Heat Treating of Ferrous Alloys,” in Steel Heat Treating Technologies, vol. 4B, J. L. Dossett and G. E. Totten, Eds., Materials Park, OH: ASM International, 2014, pp. 255-280.
- M. Ahmadin and D. K. Pratiwi, “Effect of Cooling Water Fraction of Salt in Low Carbon Steel Properties,” Journal of Mech. Sci. Eng, vol. 2, no. 1, pp. 1-5, 2015.
- L. W. Pietrasz, Zakajocznyje sriedy, Moskwa: Maszgiz, 1959.
- J. C. Hughes, “Testing of Hydrometers,” U.S. Government Printing Office, Washington D.C., 1954.