
From a metallurgical and quality perspective, aluminum heat-treating presents unique challenges when compared to carbon-based steels. This article will examine the aspects of aluminum heat-treating that make it unique, and specific ways a quality team can account for the variables to ensure conformance throughout the thermal process. I will not be covering each specific aspect of the process, so excuse me if I leave out fine details.
A Brief History
In 1807, a British chemist, Humphrey Davy (1778-1829) discovered five new metals. One was aluminum. Due to aluminum’s strong attraction to oxygen, he was unable to isolate the aluminum using an electric arc. With the introduction of bauxite into the process by Henri Sainte-Clair Deville in the 1860s, Karl Bayer (1847-1904) was able to design a process that could isolate the aluminum. Fast-forward to the modern day — the standard aluminum foundry uses the Hall-Heroult process, which has been modified over decades to produce an efficient process called electrolysis.
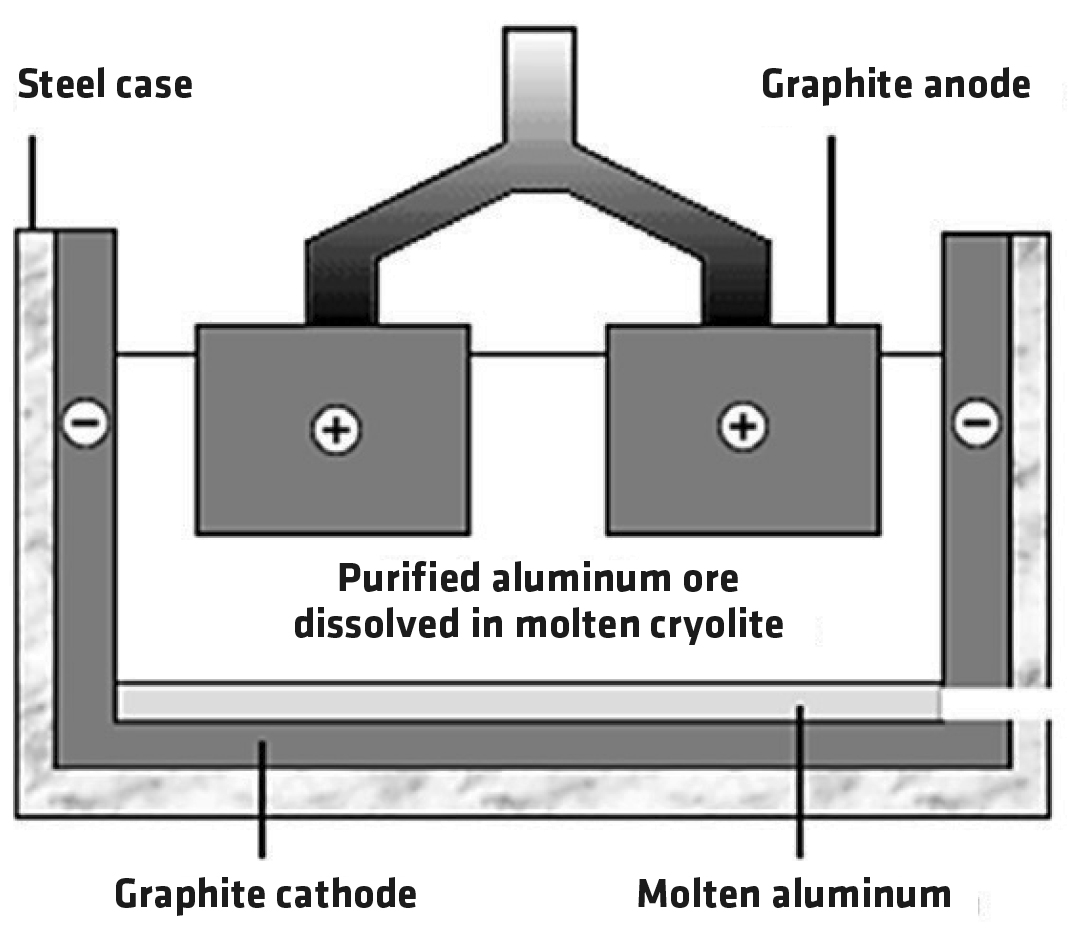
The aluminum oxide is melted and electrolyzed (Figure 1). The anode is made of graphite, a form of carbon. Oxygen ions move to the anode where they’re converted to oxygen. The anodes are gradually worn away by oxidation. The cathode is also made of graphite. Molten aluminum is produced there. The process requires a lot of electrical energy, which is one reason aluminum is more expensive than steel.
Aluminum Classification
In general, aluminum is classified as two types:
- Heat treatable (precipitation-hardenable).
- Non-heat treatable.
- Furthermore, aluminum will have a designated temper (i.e. F, O, T3, T4, T6, etc.).
Heat Treatment of Aluminum
Heat-treatable aluminum alloys can be strengthened by a suitable thermal process. The solubility of the alloy elements is directly related to temperature making wt% of each alloy element a critical factor.
Let’s use A356.0 Al alloy as an example. This alloy is made up of aluminum (primary) – 6.5-7.5% silicon – 0.25-0.45% magnesium – 0.20% copper – 0.20% iron – 0.20% titanium – 0.10% zinc. The addition of magnesium is key. Magnesium (Mg) – Al-Si- alloys that contain no magnesium are considered non-heat treatable. The addition of Mg provides solid solution strengthening without decreasing ductility. Mg additions offer strength and corrosion resistance.
In general, solution heat-treating takes advantage of the precipitation hardening reaction. The objective is to take into solid solution the maximum practical amount of the soluble hardening elements in the alloy. This process also consists of soaking the alloy at a temperature sufficiently high and for a long enough time to achieve a nearly homogeneous solid solution.
We will stick with A356.0 as we continue. Solution heat-treating of A356 produces the following effects:
- Dissolves Mg2Si.
- Homogenizes the aluminum.
- Changes in morphology of eutectic silicon.
From a quality perspective, let’s look at only the first one. To obtain the maximum concentration of magnesium and silicon, the solution temperature must be as close as possible to the eutectic temperature; ideally 10-15°F below the eutectic temperature. Control of temperature is critical. If the melting point is exceeded, incipient melting (localized melting at the grain boundary) may occur, and mechanical properties may suffer. This condition is only detectable by metallographic examination and is irreversible.
After quench, the aluminum may be aged. The process of aging causes the decomposition of various phases as the atoms dissolve in the aluminum matrix.
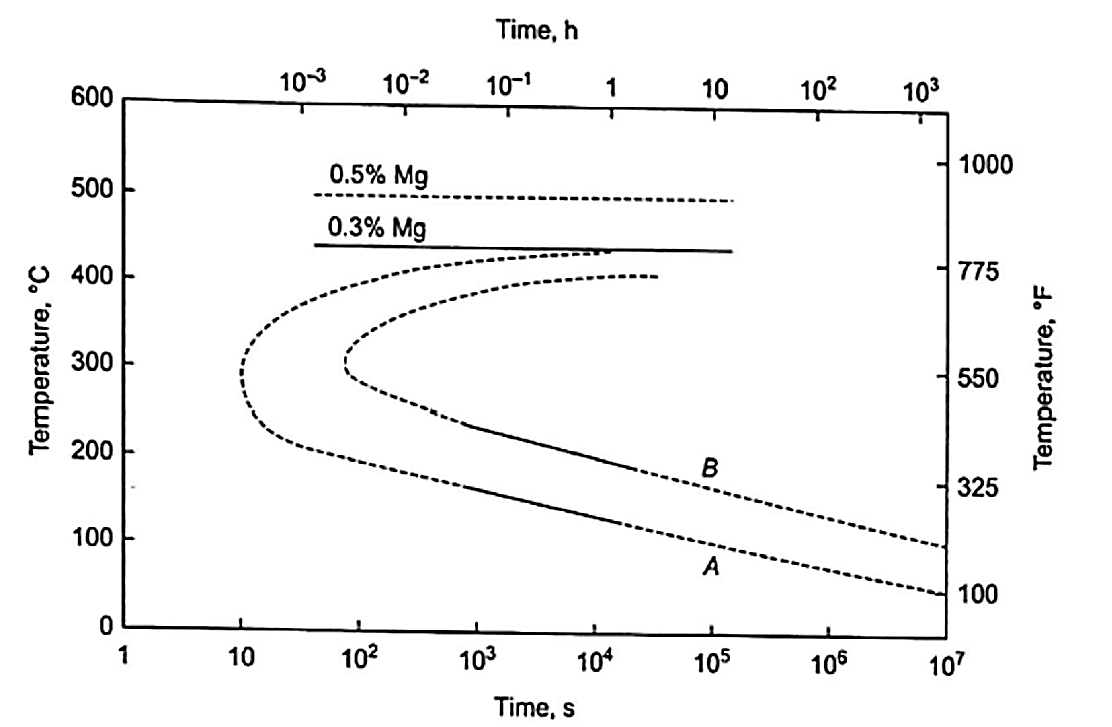
In other words, Mg2Si precipitates out of solution (which was dissolved during solution heat treatment) in order to obtain hardening characteristics (hardness and conductivity). Figure 2 is a time-temperature plot for A356.0. Line A represents the time at which Mg2Si precipitation begins. Line B represents the point at which maximum strength and hardness are achieved. An 18°F change in aging temperature changes the aging time by a factor of two.
How this Technical Description Affects Quality Characteristics
In relation to the previous information, a critical take-away should be the verification of raw material certifications. Quality personnel should design a system that will ensure all raw material received conforms to the purchase order requirements. This should include verification of each alloy element wt% as stated on the certification.
Another take-away should be solution temperature is typically 10°F-15°F below the eutectic temperature of the material. Therefore, aluminum solution heat-treating is required to take place in a furnace that has a uniformity of ±10°F (CL2 – AMS2750). When quality control is reviewing furnace charts to ensure conformance, the temperature achieved during processing is critical.
Summary
It can be difficult at times for quality-control personnel to both ensure quality requirements are met and have a thorough understanding of the process itself. Understanding the technical aspects of any thermal process will better enable the quality representatives to ensure conformance.






















