
In this article, I will discuss the transformation of pearlite and ferrite to austenite during the heating of steel into the austenite region. This is a fundamental process in the heat treatment of steels, directly influencing the final microstructure and mechanical properties.
Introduction
Pearlite is a lamellar mixture of ferrite (α-Fe) and cementite (Fe3C), typically formed during the slow cooling of austenite in steels. Ferrite is a relatively soft, ductile phase with a body-centered cubic (BCC) structure, while pearlite provides a balance of strength and ductility due to its composite nature [1]. In hypo-eutectoid steels, the microstructure at room temperature often consists of a mixture of ferrite and pearlite. In hyper-eutectic steels, the microstructure consists of a mixture of pearlite and cementite. These microstructures are shown in Figure 1.
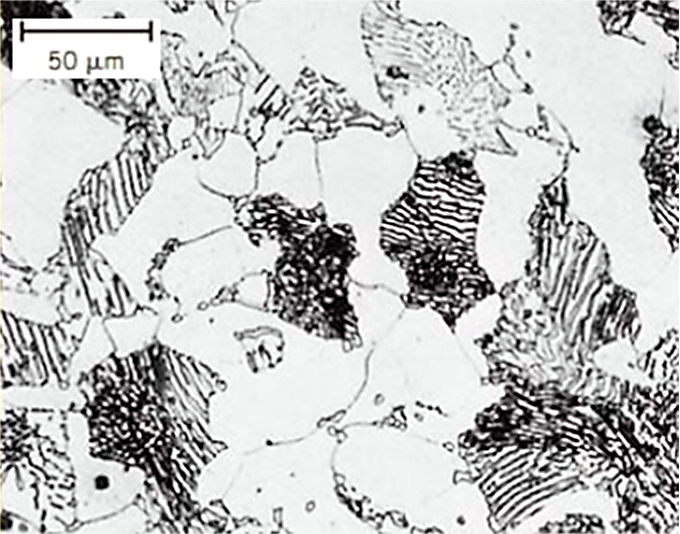
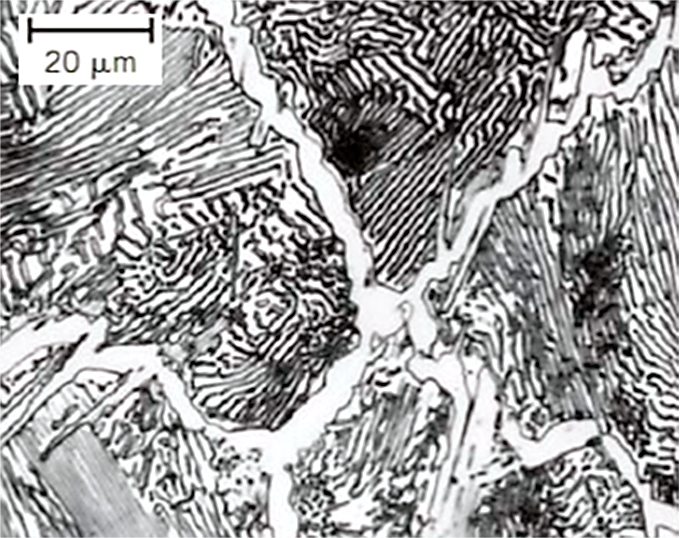
Austenitizing is the process of heating steel into the temperature range where austenite (γ-Fe, face-centered cubic structure) is stable. This transformation is thermodynamically driven and involves the dissolution of cementite and the redistribution of carbon atoms [2].
Upon heating above the eutectoid temperature (Ac1), pearlite is the first constituent to transform. This transformation is rapid due to the fine interlamellar spacing, which facilitates carbon diffusion from cementite into the surrounding ferrite, enabling the nucleation and growth of austenite at the pearlite/ferrite interfaces [3].
After pearlite is consumed, the remaining ferrite transforms more slowly. Austenite nucleates at ferrite grain boundaries and grows by absorbing ferrite, with the transformation rate governed by the diffusion of carbon and alloying elements [3].
The critical temperatures AC1 and AC3 are affected by alloying content of the steel [2]:
![]()
![]()
The critical temperatures decrease with additions of austenite-stabilizing elements (carbon, nickel, and manganese), and increase with ferrite-stabilizing elements (silicon, chromium, and molybdenum).
Austenite is not stable below Ac1; only ferrite and cementite exist. Between Ac1 and Ac3 austenite coexists with ferrite (hypo-eutectoid steels) or cementite (hyper-eutectoid steels). Above Ac3 only austenite is stable. Processing temperatures are typically chosen that are 15°C (25°F) above Ac3.
Transformation Kinetics
The kinetics of austenite formation during heating are described by the Johnson-Mehl-Avrami-Kolmogorov (JMAK) or simply Avrami equation, which models the fraction transformed as a function of time and temperature. The general isothermal form is:
![]()
where x is the volume fraction of austenite formed; K is the temperature dependent rate constant; t is time, and n is the Avrami exponent, related to the nucleation and growth mechanisms [4]. For austenite transformation under isothermal conditions, the value of n is typically between 1.5 and 3 [5].
The rate constant K is an Arrhenius-type temperature dependence:
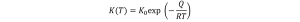
where Q is the activation energy (typically 120-170 kj/mol [6]), R is the gas constant, and T is the temperature (K).
In general, finer pearlite and ferrite structures accelerate austenite formation due to shorter diffusion distances for carbon [6] [2]. Spheroidized cementite (rounded particles) dissolves more slowly than lamellar cementite, retarding austenite formation [2].
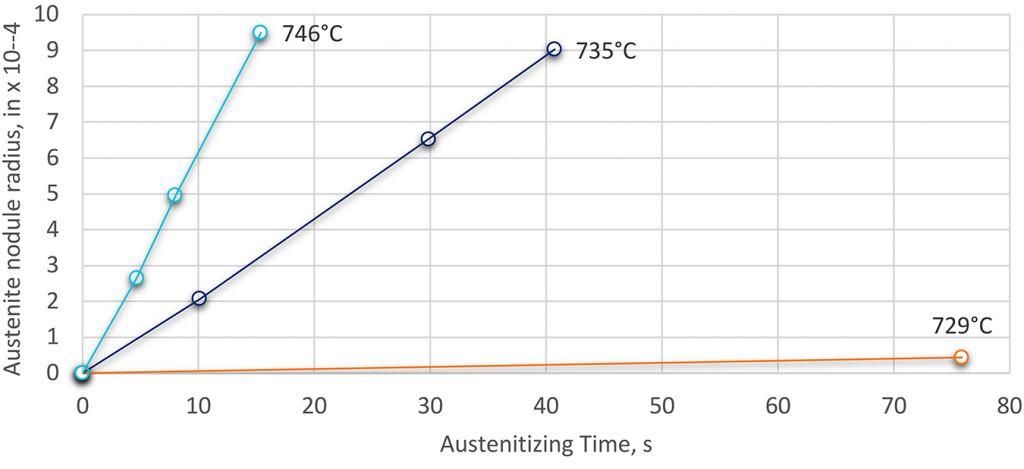
As the temperature increases, the rate of transformation also increases (Figure 2). Slow heating allows for more uniform transformation and minimizes retained non-austenitic phases. Faster heating rates shift transformation temperatures upward and can lead to incomplete transformation if the holding time is insufficient for diffusion-controlled processes [3] [2] [7].
Conclusion
In this article, I discussed the transformation of austenite from pearlite and ferrite. This is an important step in the heat-treating process. I discussed the effect of alloying elements, and the basic kinetic mechanism.
Should you have any questions or comments, regarding this article, or suggestions for further articles, please contact the writer or the editor.
References
- G. Krauss, Steels — Processing, Structure, and Performance, 2nd ed., Metals Park, OH: ASM International, 2015.
- J. G. Speer and R. J. Gaster, “Austenitizing in Steels,” in ASM Handbook: Steel Heat Treating Fundamentals and Processes, vol. 4A, J. Dossett and G. E. Totten, Eds., Metals Park, OH: ASM International, 2013, pp. 309-316.
- B. Pawlowski, P. Bala and R. Dziurka, “The effect of alloying elements on the temperature range of pearlite to austenite transformation in low alloy hypoeutectoid steels,” in Proceedings 24th International Conference on Metallurgy and Materials, June 3rd – 5th 2015, Brno, Czech Republic, 2015.
- N. Rajakrishnam, M. Sadeghifar, P. Bhattacharjee, H. Champliaud and M. Jahazi, “Kinetics of Austenite Formation in a Medium-Carbon, Low-Alloy Steel with an Initial Martensite Microstructure: Influence of Prior Austenite Grain Size,” J. Manufacturing and Materials Processing, vol. 9, no. 1, p. 10, 2025.
- E. López-Martínez, H. J. Vázquez-Gómez and B. Campillo, “Effect of initial microstructure on austenite formation kinetics in high-strength experimental microalloyed steels,” Int. J. Miner. Met. Mater., vol. 22, pp. 1304-1312, 2015.
- F. Caballero, C. Capdevila and C. Garcia de Andres, “Modelling of Kinetics of Austenite Formation in Steels with Different Initial Microstructures,” ISIJ International , vol. 41, no. 10, pp. 1093-1102, 2001.
- S. Sharma, T. Nanda, M. Adhikary, T. Venugopalan and R. Kumar, “A simulation study of pearlite-to-austenite transformation kinetics in rapidly heated hot-rolled low carbon steel,” Materials & Design, vol. 107, pp. 65-73, 2016.






















