
While some heat treatments are used to soften the material or improve its machinability, most are processed to obtain strengthened or hardened properties. The majority of heat treatments apply to metallic materials and, typically, the techniques include annealing, normalizing, quenching, tempering, precipitation strengthening, surface hardening, and case hardening. Heat treatment is so critically important that we can safely say a part undergoing extensive manufacturing processes such as melting, rolling, forging, and other related machining is of little or no value without the necessary and appropriate heat treatment.
Carburizing is one of the most widely used case hardening treatments. Per AGMA 923, carburizing is defined as a heat treatment process in which an austenitized steel is brought into contact with a carbonaceous atmosphere of sufficient carbon potential to cause adsorption of carbon bearing gases at the surface where they dissociate and, by diffusion, to create a carbon concentration gradient [1].
Carburizing is generally followed by quenching and tempering. After quenching, the outer surface becomes harder via martensitic transformation due to its higher carbon content, while the core remains relatively soft and tough. Tempering is performed to increase the toughness and ductility of the quenched part. Through carburizing and quenching plus tempering the part, the results are increased surface hardness, wear resistance, fatigue, and tensile strength, as well as the desired compressive residual stress on the surface. Consequently, the part also experiences grain growth and distortion.
Carburized parts are so popular that they are used in almost every industry including aerospace, transportation, power transmission, manufacturing, and material processing. In the automobile industry, the global output of gears in 2014 was estimated to be approximately 1 billion, and most of them are carburized [2].
Per definition, carburizing material is usually low-carbon steel, normally with a carbon content of ≤ 0.25 wt.%. In the past, plain carbon steels, such as SAE 1020, were used; however, with the demand of carrying a heavier load, more alloy steels have been developed and carburized, such as SAE 4320, 8620, and 9310 and 17CrNiMo6/18CrNiMo7-6, 16MnCr5, and 20MnCr5. Carburizing temperature usually lies in the range of 900°C to 950°C. Surface hardness is in the range of 55 HRC to 64 HRC. Case depth can be anywhere between 0.4 mm and 9 mm. A very thick case can carry heavy torque and stress, which is useful in specific applications such as marine and sugar mill gears.
Historically, there are three types of carburizing methods depending on the carbon source: solid carburizing, liquid carburizing, and gas carburizing. Charcoal, molten salt, and carbon-bearing gases, such as natural gas and propane, are used correspondingly. Among them, gas carburizing is the most common type and provides precise and homogeneous control of case depth with economical and cost-effective benefits. Today, most of the world’s carburizing procedures are accomplished with gas carburizing. This includes both atmosphere and low pressure (vacuum) carburizing. Although vacuum carburizing is becoming more popular, atmosphere carburizing is still the most common gas carburizing procedure. (See Figure 1 for an example of atmosphere gas carburizing.)

Although atmosphere carburizing consists of several process steps [3], it can be simplified to include two major processes: carbon generation in the furnace and carbon diffusion into the workpiece. The former provides carbon atoms while the latter determines the carbon concentration gradient.
It should be noted that gas carburizing is a complicated procedure during which many chemical reactions occur simultaneously in the carburizing atmosphere. The most commonly used carbon-source gas is natural gas (methane, CH4), while the endothermic gas is the preferred and most widely used carrier gas, which is usually produced by mixing air and natural gas in a fixed proportion, usually a 2.5-5 to 1 ratio. Reactions take place within the gas mixture when it passes through a chamber with a catalyst, e.g., NiAl. As a result, endothermic gas is composed of nitrogen (N2), carbon monoxide (CO), carbon dioxide (CO2), hydrogen (H2), water (H2O), and methane (CH4), and enters the furnace together with carbon source gas. Among them, CO and CH4 are carburizing agents, while CO2 and H2O are decarburizing agents. It is estimated that there are over one hundred reactions inside the carburizing atmosphere. However, the following three reactions are the most important and determine the rate of carbon transfer from the carburizing atmosphere to the steel surface [4]:
2CO ↔ C(γ-Fe) + CO2 Reaction 1
CH4 ↔ C(γ-Fe) + 2H2 Reaction 2
CO + H2 ↔ C(γ-Fe) + H2O Reaction 3
Reaction 3 is about two orders of magnitude faster than the other two, therefore it determines the rate of carbon adsorption during the process [5].
The balancing reaction for gas carburizing atmosphere is called water-gas reaction and can be expressed by:
CO + H2O ↔ CO2 + H2 Reaction 4
Typically, these four main reactions determine the carbon potential that provides carbon atoms for carburizing. After carbon potential is detected, a furnace controlling system adjusts the gas ratio to reach the target. Once the atoms are generated and adsorbed on the surface, they diffuse into the workpiece. The diffusion velocity depends on temperature, carbon potential in the atmosphere, and chemical composition of the steel. This step takes up the majority of time in the carburizing cycle. For example, if we want to get a very thick case, e.g., 0.35″ (9 mm), an SAE 9310 steel part needs to be in the carburizing furnace for more than 10 days when carburized at 940°C. To obtain a desired homogeneous carburizing case depth, it is imperative to keep all carburizing parameters (temperature, carbon potential, and cycle time) under control. In addition to the surface hardness and case depth, other characteristics — such as surface carbon content, core hardness, and microstructure (retained austenite, carbide distribution, etc.) — should also meet the related requirements.
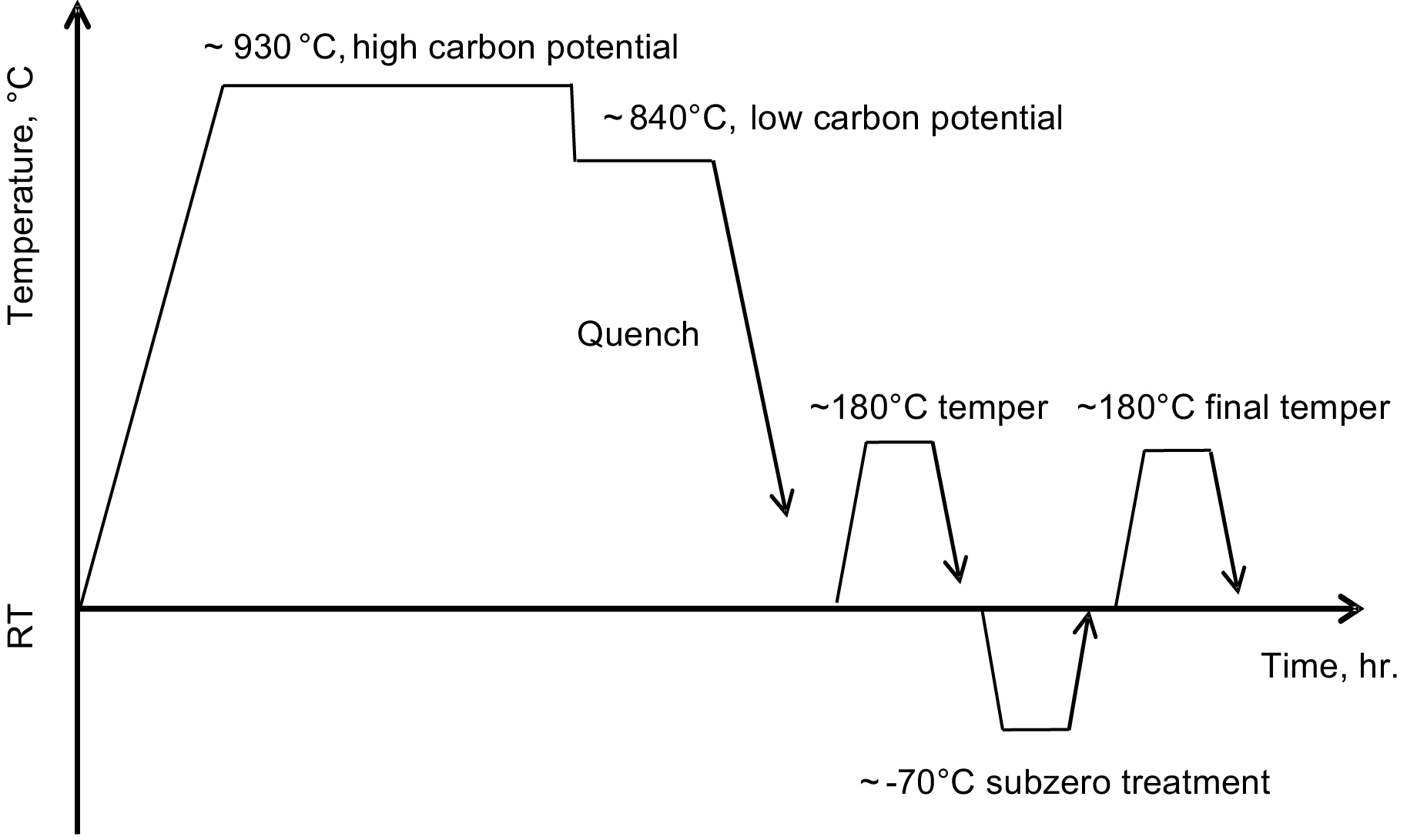
After carburizing, the part is quenched into the appropriate quenchant (water, oil, or polymer solution), followed by tempering, typically around 180°C. Keep in mind that not all austenite transforms into martensite after quenching. If the retained austenite is within 30 percent of the product phases, the carburized and hardened part can then move to the next manufacturing processing. Otherwise, a sub-zero treatment is needed, during which retained austenite transforms into martensite so that its fraction is no higher than 30 percent. SAE 9310 is a good example for this treatment. After that, the part needs to be tempered again. Figure 2 illustrates a carburizing cycle.
References
- AGMA 923-B05, Metallurgical Specifications for Steel Gearing, American Gear Manufacturers Association, 500 Montgomery Street, Suite 350, Alexandria, Virginia 22314. p. 11.
- Maciej Korecki, Emilia Wolowiec-Korecka, Doug Glenn, Single-Piece, High-Volume, Low-Distortion Case Hardening of Gears, AGMA Fall Technical Meeting, October 18-20, 2015, Detroit, Michigan.
- Aymeric Goldsteinas and Rene Alquicer, Producing Quality Parts in an Atmosphere Furnace: How to Optimize Your Quenching and Carburizing Processes, Thermal Processing for Gear Solutions, April 15, 2015, p. 30.
- R. Collin, S. Gunnarson and D. Thulin, Mathematical Model for Predicting Carbon Concentration Profiles of Gas-Carburized Steel, Journal of the Iron and Steel Institute, 210 (10), 1972, p. 785.
- Olga Karabelchtchikova, Fundamentals of Mass Transfer in Gas Carburizing, Ph.D. thesis of Worcester Polytechnic Institute, November 2007, p. 7.























