There have recently been several inquiries regarding the use of a polymer quenchant in a sealed or integral quench furnace. There are several drivers for this — cost, for example. While the polymer quenchants have a higher-per-gallon cost than oil quenchants, the in-use concentration of polymer quenchants is much less than an oil. There are also environmental issues. Polymer quenchants do not give off as much smoke as oil, and disposal is easier. The use of a polymer quenchant in a sealed quench furnace can be done, but it requires some special design elements. (Figure 1)
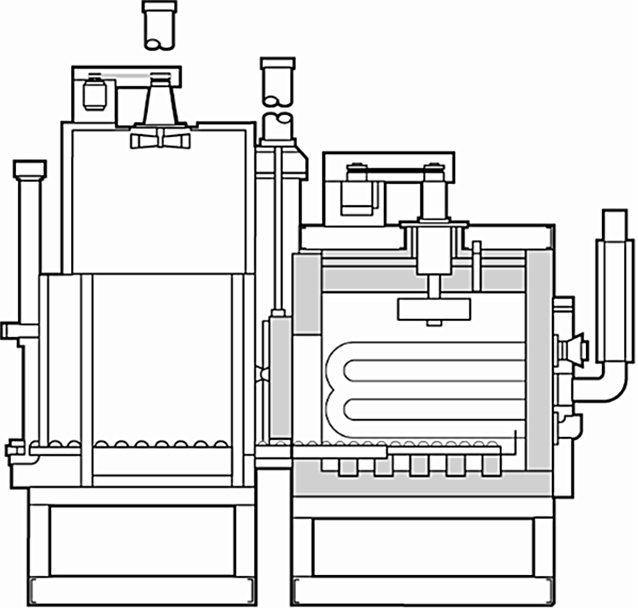
First, there is the hot zone. The inner door needs to be completely sealed to prevent water vapor from infiltrating the hot zone and increasing the chance for creating a decarburizing atmosphere. It is possible to use a much greater volume of atmosphere in the hot zone, creating a higher partial pressure of atmosphere in the hot zone. As far as I know, most manufacturers prefer to seal the inner door to prevent water vapor entering the hot zone.
Gas introduction
Since the hot zone is sealed, it is often necessary to introduce nitrogen or other inert gas into the vestibule. This prevents the parts from oxidizing during part transfer into the quench and helps purge oxygen from the vestibule. Using an endothermic atmosphere can be done, but there is a much greater chance of explosion since the vestibule is below 760°C. Nitrogen is probably the easiest solution.
Lastly, there is the quench tank. When quenching in oil, the allowed maximum peak operating temperature during quenching is approximately 50°F (28°C) below the flash-point temperature. There is an additional 50°F (28°C) as a safety factor for hung loads, partially submerged loads, stuck elevator, etc. Using the “one pound per gallon of oil” rule of thumb, the temperature will rise about 70°F (39°C). This also determines the maximum operating temperature of the oil. For an oil with a flash point of 350°F (177°C), the maximum peak temperature during quenching would be 250°F (121°C) from subtracting the regulatory required 28°C, and the additional 28°C safety factor (177°C flash point — 28°C regulatory difference — 28°C additional safety factor = 121°C). Since the maximum peak temperature during quenching is governed by the “one kilogram to eight liters” (one pound per gallon) rule, or a 70°F or a 39°C temperature rise, the maximum operating temperature of the oil is 82°C and the maximum peak temperature during quenching is 121°C.
Polymer quenchants
For polymer quenchants, the design rules are completely different and are based on different criteria. There are also different criteria based on different types of polymers. Looking at PAG-type polymers, these polymers have a distinct cloud temperature and are inversely soluble. The cloud temperature is where the polymer precipitates from the water. Inversely soluble means that the solubility of the polymer decreases as the temperature increases. Depending on the PAG polymer, the cloud temperature can range from 57°C to 82°C. Using the higher cloud temperature of 82°C (most common PAG type polymers), this establishes the “do not go past temperature” like flash point. However, there is no safety hazard associated with this temperature, just an extremely high drag-out of the polymer, a sticky mess, and excessive oxidation of the product. Therefore, you need some sort of safety factor for the temperature rise. Most people limit the peak temperature during quenching to a maximum temperature of 60°C (140°F). This temperature is a good compromise between excessive drag-out and oxidation of the polymer.
Other polymers have different criteria. For PVP-type polymers, there is no distinct cloud point, because it is above the boiling temperature of water. It is also inversely soluble. However, the maximum temperature is higher. Some people use 65°C-70°C as the maximum peak temperature during quenching, while others use a lower temperature. Also remember that the ability of the polymer to extract heat decreases as the temperature increases. As temperature decreases, the quench rate slows down. Because of this, often a much lower concentration of polymer is used if a higher allowable temperature rise is permitted. In continuous-type furnaces, where there is a quench chute, roughly half the concentration or less is needed because of the local temperature rise in the quench chute. However, use of a polymer quenchant in a continuous furnace has its own issues.
This allowable temperature rise establishes the necessary quench tank size for a given workload size. In general, it takes about twice the volume in polymer as the volume of oil necessary. In other words, if a given sealed quench furnace can handle 1,000 kilograms of product using oil, the same furnace would have a load reduction of 50 percent, or about 500 kilograms, for the same size quench tank.
Additionally, polymer quenchants are more sensitive to agitation rates than oils. In general, polymers will require more agitation than oils. The amount is hard to quantify. Uniformity is the biggest issue.
Quench cooling
Lastly, cooling of the quench is important for the next load. For oil quenchants, the quenchant is easily cooled with an air-oil heat exchanger, since the operating temperature is quite a bit higher than the ambient temperature. For polymer quenchants, since the operating temperature is near ambient temperature, the efficiency using an air-to-polymer heat exchanger is quite low. Additional cooling using a cooling tower and chilled water is necessary. This creates additional cost for installation.
While the use of a polymer quenchant is much more environmentally friendly, safer, and the in-tank cost is much lower than oil, the reduced workload size; the necessity of chilled water to cool the quenchant; and the cost of modifying the furnace to be compatible with polymer quenchants, tend to nullify the benefits of the use of polymer quenchants in a sealed quench furnace. It can be done, and has been done successfully, but you must understand the limitations.


































