
Polyalkylene glycol quenchants are used in a variety of different heat-treating applications. One very common application is quenching of large steel and aluminum components. These are often large. open tanks and exposed to the air. The polymer is usually diluted with city water or well water. This water can contain different cations such as calcium, magnesium, sodium, and anions like carbonate, chlorides, or phosphates. As the solution of water/polymer is used, additional make-up water is added to replenish the water lost to evaporation. However, the salts present continue to build-up in the bath. They are not lost to evaporation. As these salts build up in the bath, the factor used for determining concentration using a handheld refractometer will change. Eventually, the salt content reaches a threshold where it becomes difficult to accurately control concentration. The decision must be made whether to dump and recharge the system. For small induction units, where the total system volume is 400 liters, and the concentration is low (5 percent or so), it is usually the most cost-effective to simply dump the system and refill it. For a larger system, containing 20 percent PAG and a large quench tank, it is a large cost to dispose and recharge a system. Alternatively, it is possible to recycle and reclaim the polymer, while disposing the contaminated water.
Theory
Polyalkylene glycol quenchants undergo a phase separation as the solution temperature is raised. At a given temperature, the thermal energy of the system becomes greater than the energy of the water-polymer hydrogen bonding interactions. When this occurs, the polymer loses its solubility in water. A two-phase solution develops, with the water in the upper layer, and the heavier polymer in the bottom layer. This is not a perfect separation, as the upper layer will be water-rich, and the lower layer will be polymer-rich. The temperature at which separation occurs is called the separation temperature or the cloud point. This is illustrated in Figure 1. The separation temperature of the various PAG polymers is shown in Table 1.
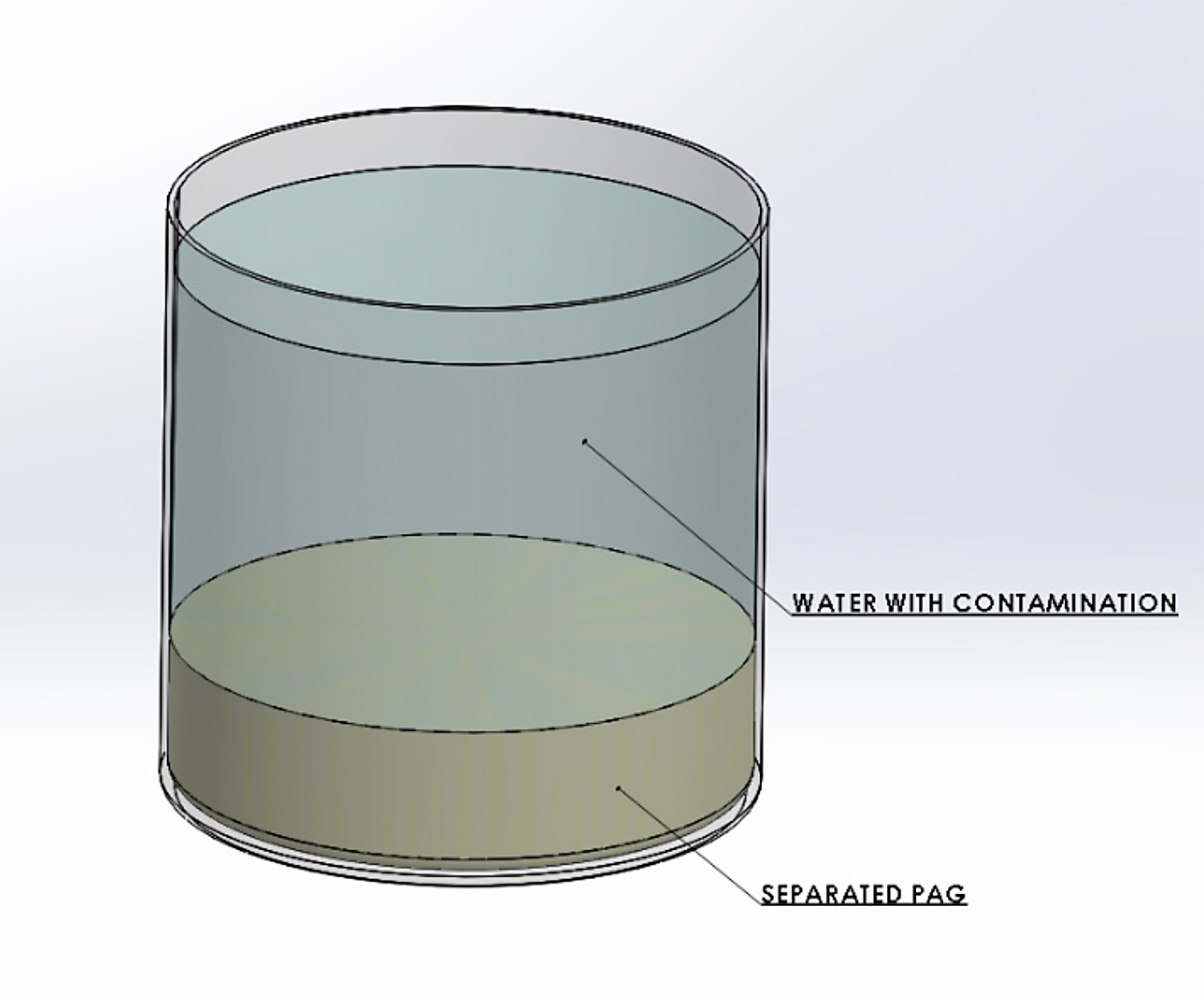

The cloud point or separation temperature is often used to purify or clean up the quenchant bath from impurities and contaminates. Typical impurities include salts from the water supply, or from salt-bath heat-treating drag-out. To accomplish this, the polymer solution is raised in temperature to separate the PAG and water. After separation and layer formation, the aqueous portion is removed. The inorganic salts, being ionic, report to the aqueous phase. The purified polymer, which forms on the bottom layer, is then re-dissolved with fresh water to the desired concentration or is used neat as make-up for controlling the concentration of the bath. However, if the salt concentration is too great, (typically greater than 10 percent), then the water/polymer separation is reversed: i.e., the water reports to the bottom, and the polymer reports to the top. This is really a function of the specific gravity of the water-salt solutions, and the specific gravity of the polymer.
General Procedure
While there are many variables related to polymer recovery, and these can change at each customer’s location, some general procedures and recommendations can be made. Separation can take place in either separate heated holding tanks or in the quench tank. Both situations are commonly encountered. The overall procedure is as follows:
Filter the PAG-water solution to remove the particulate matter. A recommended filtration media for PAG solutions is a sand-type filter.
After filtration, heat the PAG-water solution to approximately 15°F (8°C) above the cloud point of the polymer (Figure 1). The solution should be agitated thoroughly. Care must be taken not to burn the solution on the heating elements. It is recommended that a maximum watt density of 6-10 watts per inch be used on the heating elements.
Once the desired temperature has been reached, the agitators are turned off, and the solution allowed to settle. Typically, this may require one to two hours, depending on the size of the quench tank, and the concentration of the PAG. The use of a water-soluble dye in the solution will aid in determining the boundary of the two phases. In addition, the use of a clear sight glass will also aid in separating the water and polymer. Agitation should remain off, and the solution should be maintained at the recovery temperature.

After adequate time has passed to effect separation, the water is pumped out of the tank, or the polymer is drained from the tank. Either is acceptable, and will depend on the individual situation. Most commonly, the contaminated water is pumped out. This salt and contamination laden water is disposed of according to local, state, and federal regulations.
The remaining recovered polymer can then be diluted and used as make-up, or used neat as make-up.
The most common corrosion inhibitors are inorganic salts such as sodium nitrite. These salts provide corrosion protection by passivation of the surface. Because they are aqueous inorganic salts, they report to the separated water phase, depleting the polymer of the corrosion inhibition. The remaining polymer should be evaluated for the presence of rust inhibition. Should inadequate amounts be present, and then appropriate additions of rust inhibitor should be made. The Houghton Heat Treating Laboratory can help determine the proper concentration of rust inhibitor required, and the proper type.
Typically, for nitrite-type corrosion inhibitors, the use of 0.5-2 percent of the recommended inhibitor is recommended. For amine type corrosion inhibitors, the use of 1-3 percent of amine type inhibitor is recommended. It is very important that the different corrosion inhibitors not be mixed.
Conclusions
In this short article, we described the basic reasons for performing a thermal separation of a polyalkylene glycol solution, and the basic procedure. The economics of performing the thermal separation depend on the cost of energy to heat up the solution, disposing the separated water, and the cost of replenishing the corrosion inhibitors. This should be compared to the cost of disposing the entire system and filling the system with the polymer quenchant.
Should there be any questions regarding this article, or suggestions for further articles, please contact myself or the editor.























