
In this column, I will discuss the intergranular corrosion of austenitic stainless steels (3XX) and discuss the thermal processing causes.
Introduction
Austenitic stainless steels, such as 304, are used in practically every industry, for chemical plants to kitchen appliances. They are widely used because of their attractive appearance and resistance to corrosion, especially in wet environments.
Austenitic stainless steels are a group of alloys that contain approximately 18 percent chromium and 8 percent nickel. Other alloying elements such as molybdenum and titanium are added to enhance corrosion resistance. Typical compositions of austenitic stainless steels are shown in Table 1. Molybdenum is added to increase overall corrosion resistance, while titanium is added to the melt to trap carbon and form titanium carbides. Typically, titanium is added at five times the amount of carbon and nitrogen present in the melt. Titanium is a strong carbide former, and is a stronger carbide former than chromium, and minimizes the formation of chromium carbides. Sulfur is added to AISI 303 stainless steel to improve the machinability and chip formation.
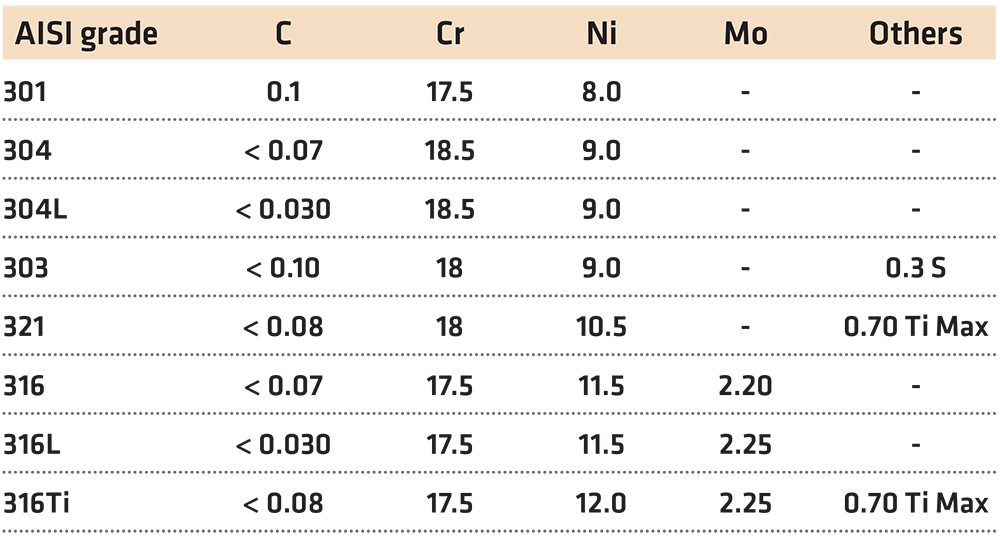
The “L” designations after the alloy number means that the carbon content is controlled to a much lower level (< 0.030 C). This is to improve weldability and to minimize chromium carbide formation during welding.
Sensitization of Austenitic Stainless Steels
When austenitic stainless steels are heated in the temperature range of 450 – 900°C, they become sensitized to intergranular corrosion. In many test methods, deliberate sensitization of austenitic stainless steels is accomplished by deliberately holding it at 650°C for one to two hours [1]. Sensitization can occur by holding the alloy at this temperature, or slow cooling the alloy through this temperature range (annealing or slow cooling after welding).
In the temperature range of 450-900°C, carbon, and chromium carbide, Cr23C6, is insoluble in the matrix and precipitates out of solid solution. This occurs when the carbon content is greater than approximately 0.02 percent. The chromium carbide precipitates at low energy sites such as grain boundaries. This removes chromium from the matrix and creates a depleted chromium region adjacent to the grain boundary. This is schematically shown in Figure 1. This effect is striking when viewed metallographically (Figure 2).
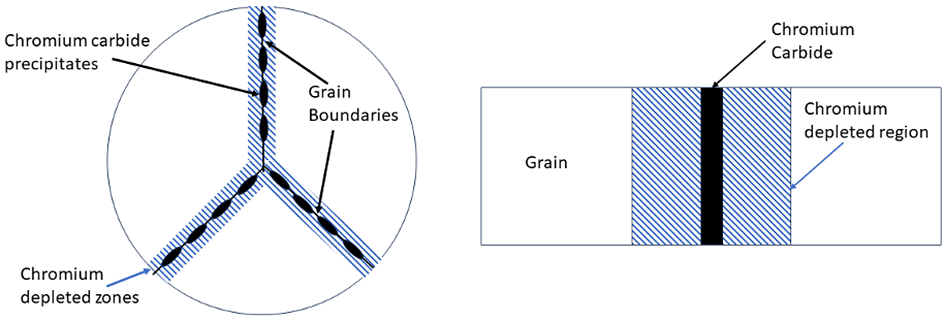
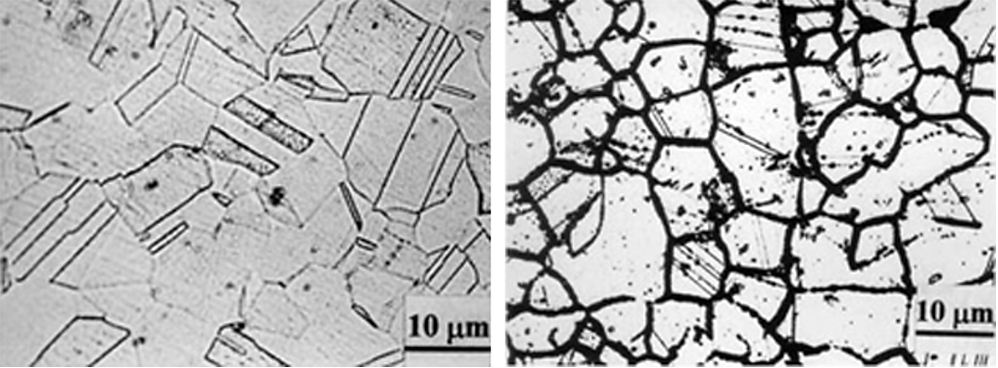
In a wet chloride environment, or other acids with low pH, the chromium-depleted regions do not have adequate chromium present to provide sufficient corrosion protection. This chromium-depleted region is preferentially attacked, while the interior of the grain and the carbide is unaffected [3]. The interior of the grain and the chromium-depleted zone are in intimate contact, with the grains having a much larger area than the grain boundaries, resulting in dissimilar metal compositions. The chromium-depleted regions protect the interior of the grains. A rapid attack of the chromium-depleted region occurs.
This slow cooling of a part through the sensitization zone can often occur in welding of austenitic stainless steels, especially in the HAZ, or Heat Affected Zone. This occurs when the weld slow cools through the critical temperature range of 450-900°C, with precipitation of chromium carbide at the grain boundaries. The HAZ spends greater time in this critical range, and preferential corrosion of the HAZ can occur. This phenomenon is called “weld decay.”
Austenitic stainless steels are not hardened by heat treatment. Only by cold work do these steels increase hardness. After processing, usually at the mill, austenitic stainless steels are solution process annealed around 1,050°C to dissolve carbides and any sigma phase present. These carbides may form during heating to the solution annealing temperature. A rapid quench, usually in water, follows solution annealing. This process prevents the formation of carbides at grain boundaries and minimizes the possibility of the chromium-depleted zone surrounding the carbides.
Carbide formers, such as titanium, niobium, and tantalum are used to prevent the formation of chromium carbides. These elements are much stronger carbide formers than chromium, so scavenge residual carbon.
 Testing for Sensitization
Testing for Sensitization
The predominant test methods for determining if an austenitic stainless steel is sensitized is ASTM A262 [1]. The test specification describes several testing methods to understand if the alloy is susceptible to intergranular corrosion, or if any thermal processing has created a sensitized structure. Often, this is done as part of an initial qualification testing of the part or process [4].
ASTM A262 Practice A – “Oxalic Acid Test”
In Practice A, a test panel is metallographically prepared and examined for the presence of steps or “ditches.” The sample is electrolytically etched with 10 prcent at a current density of 1A/cm2 for 90 seconds. This is a quick test and is often used for screening materials.
The microstructure revealed is compared to a series of photomicrographs in the standard, and the degree of sensitization.
ASTM A262 Practice B – “Streicher Test”
In this test, a sample panel is immersed in boiling solution of ferric sulfate and 50 percent sulfuric acid for 120 hours. The weight loss of the sample is measured and then converted to a corrosion rate. This is compared to a reference corrosion rate that is not expected to be prone to intergranular corrosion.
ASTM A262 Practice C – “Huey Test”
This method involves subjecting the sample to a series of five periods of boiling nitric acid of 48 hours each period, with a new solution after each period. This test is not performed often because of the time involved, and potential attack of other phases.
ASTM A262 Practice E – “Strauss Test”
The Strauss test was the first test used for detecting sensitized steels, and susceptibility to intergranular corrosion. It is a pass-fail test.
In this method, the sample is exposed to a boiling solution of copper shot, copper sulfate, and 16 percent sulfuric acid for 15 hours. After the exposure, the sample is bent at 180 degrees. The fracture surface is then examined under optical microscopy at low magnification. If fissures or cracks appear, the presence of intergranular attack is confirmed.
ASTM A262 Practice F
This method is very similar to Practice E, but metallic copper is not used. Higher concentrations of copper sulfate and sulfuric acid is used. Weight loss, after 120 hours, converted to corrosion rate, is used to determine resistance to intergranular corrosion.
Conclusions
In this article, I have described the cause of intergranular corrosion in austenitic steels, and some of the steps taken to reduce 3XX stainless steels’ susceptibility to intergranular corrosion. Reducing the carbon content and adding strong carbide formers to the melt to prevent chromium carbide, are the primary mill-controlled methods.
In fabrication (or service), care should be taken to avoid exposure in the 500-800°C temperature range. This can result in the formation of chromium carbides at the grain boundaries, and the material becoming prone to intergranular attack due to sensitization.
Should you have any comments on this article, or suggestions for additional articles, please contact the writer or the editor.
References
- ASTM International, “ASTM A262-15 (2021) Standard Practices for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steels,” ASTM International, West Conshohocken, PA, 2021.
- M. G. Fontanna, Corrosion Engineering, 3rd ed., New York, NY: McGraw Hill Book Company, 1987.
- W. D. France and N. D. Greene, “Predicting the Intergranular Corrosion of Austenitic Stainless Steels,” in 21st NACE Conference, March 15-19, St. Louis, MO, 1965.
- U.S. Nuclear Regulatory Commission, “Regulatory Guid 3.37 Guidance for Avoiding Intergranular Corrosion and Stress Corrosion in Austenitic Stainless Steel Components of Fuel Reprocessing Plants,” Office of Standards Development, U.S. Nuclear Regulatory Commission., Washington D.C., 1975.
- R. H. Aborn and E. C. Bain, “Nature of the Nickel-Chromium Rustless Steels,” Trans. Amer. Soc. Steel Treat., vol. 18, pp. 837-873, 1930.






















