Editor’s note » This is the third in a five-part series.
In this third segment of my series on heat-treating techniques, I will discuss the pros and cons of gas liquid carburizing, also known as cyanide carburizing (cyaniding).
The Basics of the Process
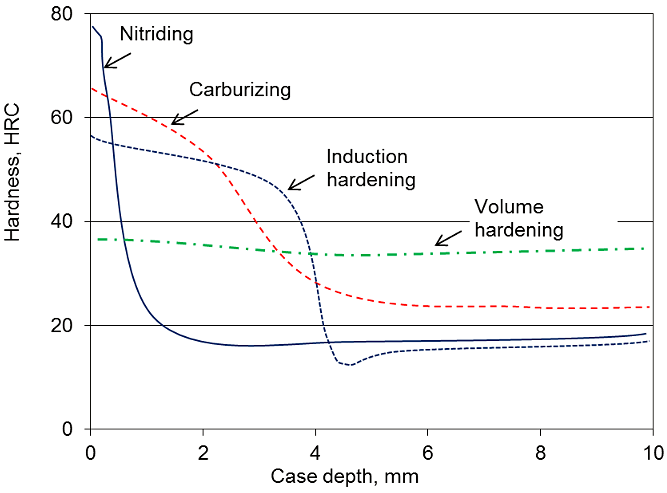
These processes produce a hard, thin layer between 0.25 and 0.75 mm (0.01 and 0.03 inches) that is similar to or harder than the one produced by carburizing. Typically, the process can be completed in 20 to 30 minutes compared to the several hours common with the case carburizing process, thus neither the internal and/or surface stresses will be relieved. Heat-treat processes that require the surface and/or body of the part to be heated above approximately 1,100°C will relieve these stresses. If these internal and/or surface stresses are not uniform, the relief of these stresses will be non-uniform, and heat treatment distortion will occur. Even if the base material is very uniform in both internal stresses (stress relieved and annealed, etc.), the process of cutting the gashes to form the gear teeth induces non-uniform stresses in the surface of the part from the shearing operation of cutting. Unfortunately, the major drawback of cyaniding is that cyanide salts are poisonous.
The difference between liquid carburizing and gas carburizing is that in gas carburizing, carbon is generated by disassociate butane, methane, propane, or natural gas. In liquid carburizing, carbon is leeched out from a molten salt composed mainly of sodium cyanide (NaCN) and barium chloride (BaCl2). In pack carburizing, carbon monoxide is driven out of coke or hardwood charcoal and then disassociated. Liquid carburizing may be distinguished from cyaniding (which is performed in a bath containing a higher percentage of cyanide) by the character and composition of the case produced. Cases produced by liquid carburizing are lower in nitrogen and higher in carbon than cases produced by cyaniding.

Liquid Carburizing
The liquid carburizing process facilitates generation of a hard, thin case on the exterior of metal parts with a high basic (internal) carbon content. It usually requires comparatively little time to complete. Liquid carburizing proves very efficient for strengthening the surfaces of small and medium-sized parts; manufacturers can automate this process easily. This is a process used for case (surface) hardening steel or iron parts. The parts are held at a temperature above Ac1 in a molten salt bath. In the supercooled austenite transformation curve of steel, Ac1 represents the critical temperature at which pearlite transforms to austenite during heating; Ac3 represents the final critical temperature at which free ferrite is completely transformed into austenite during heating. Once the temperature of the part has sufficiently stabilized, the process then infuses carbon and nitrogen, or carbon alone, into the surface of the metal part. Most liquid carburizing baths contain cyanide, which introduces both carbon and nitrogen into the case.
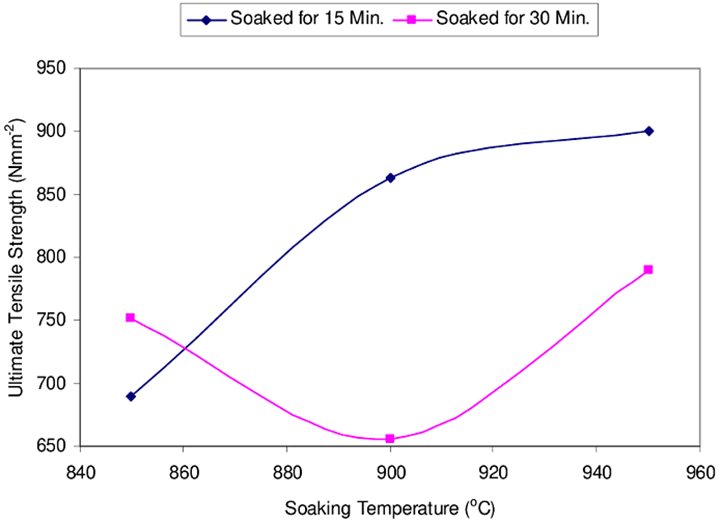
Liquid carburizing is carried out by placing the component in a salt bath at a temperature of 845 to 955 °C. The salt is usually a cyanide-chloride-carbonate mixture and is highly toxic. The cyanide salt introduces a small amount of nitrogen into the surface which further improves its hardness. Although this is the fastest carburizing process, it is suitable only for small batch sizes. Liquid or cyanide carburizing is a case-hardening process that is fast and efficient; it is mainly used on low-carbon steels.
It is a method of case hardening involving the diffusion of carbon and nitrogen into the surface layer of steel in cyanide-salt baths at temperatures of 820 to 860°C, defined as medium-temperature cyaniding, or 930 to 950°C for high-temperature cyaniding. Its principal purpose is to increase the hardness, wear resistance, and fatigue limit of steel products.
During cyaniding, the cyanide salts are oxidized with the liberation of atomic carbon and nitrogen, which diffuse into the steel. In medium-temperature cyaniding, the cyanide layer formed (containing 0.6 to 0.7 percent carbon and 0.8 to 1.2 percent nitrogen) has a thickness of 0.15 to 0.6 mm, while in high-temperature cyaniding (a method often used instead of carburizing), the cyanide layer, containing 0.8–1.2 percent carbon and 0.2–0.3 percent nitrogen, has a thickness of 0.5 to 2 mm. After cyaniding, a product undergoes hardening and low-temperature tempering.
Liquid carburizing is carried out in a container filled with molten salt, such as sodium cyanide. This bath is heated by electrical immersion elements or by a gas burner, and stirring is done to ensure uniform temperature. This process gives a thin, hardened layer up to 0.08 mm thick. Parts that are to be case-hardened are dipped into a liquid bath solution containing calcium cyanide and polymerized hydro-cyanide acid or sodium or potassium cyanide along with some salt. The bath temperature is kept between 815 to 900 °C. The furnace is usually built around a carbon steel case or heat-treatment tray, which may be fired by oil, gas, or electrically. If only selected portions of the components are to be carburized, then the remaining portions are covered by copper plating. There are some advantages of the liquid bath carburizing process as a greater depth of penetration is possible.
Cyaniding or Pack Cyanide or Pack Carburizing
Cyaniding or pack cyanide carburizing is used to case harden steel. It generates a very thin but hard outer case. Cyaniding is a case-hardening process in which both carbon and nitrogen in form of cyaniding salt are added to the surface of parts machined from low- to medium-carbon steels. Sodium cyanide or potassium cyanide may be used as the hardening medium, which is a process of superficial case hardening. It combines the absorption of carbon and nitrogen to obtain surface hardness. The components to be case hardened are immersed in a bath containing fused sodium cyanide salts kept at 800 to 850 °C. The component is then quenched in bath or water. This method is effective for increasing the fatigue limit of medium- and small-sized parts such as gears, spindle, shaft, etc.
Cyanide hardening has some advantages and some disadvantages over the case carburizing or nitriding process methods. The cyaniding process also provides a bright finish on the part. More importantly, distortion can be manageable and / or avoided and the effective fatigue limit can be increased. However, as mentioned earlier, the main disadvantage of this process is that it is costly and uses highly toxic agents in the process, which compares less favorably to other processes of case hardening.
Cyaniding or pack cyanide carburizing has the highest production rate. In this process, components are packed in an environment with high carbon content. The key difference between cyaniding and carbonitriding is that cyaniding uses sodium cyanide liquid, whereas the carbonitriding process uses a gaseous atmosphere consisting of ammonia and hydrocarbons. Moreover, cyaniding involves temperatures around 855 to 955 °C. Despite this increased complexity, gas carburizing has become the most effective and widely used method for carburizing steel parts in large quantities. The process provides a number of advantages, such as (but not limited to) high process speed and ease of control, low cost of equipment and production space, the possibility of precluding grain regeneration owing to the short duration of the cyaniding process, uniformity of heating of the part, high flexibility of the process (since parts requiring different depths of hardness may be treated in the same bath), and the possibility of direct quenching from the salt bath.
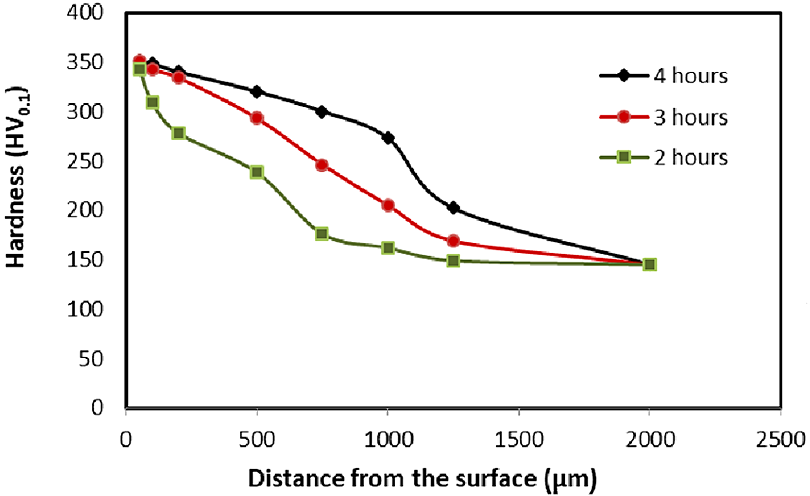
Metals to be carburized such as low carbon steel are placed in cast iron or steel heat-treatment trays containing a material rich in carbon such as charcoal, crushed bones, potassium ferro-cyanide, or charred leather (in high-performance gear applications, we tend to use a more controlled carbonizing material). Such containers or trays are made of heat-resisting steel (or other materials) which are then closed and sealed. The parts, carbon material, and trays are heated to a temperature of 900 to 950°C according to the type of steel being treated. The carbon enters the metal to form a solid solution with iron and converts the outer surface into high carbon steel. Consequently, pack-hardened steel pieces have carbon content up to 0.85 percent in their outer case. After this treatment, the carburized parts are cooled in boxes. Only plain carbon steel is carburized in this process for hardening the outer skin and refining the structure of the core to make it soft and tough. Small gears are case hardened by this process for which they are enclosed in the cast iron or steel box containing a material rich in carbon, such as small piece of charcoal, and then heated to a temperature slightly above the critical range. Depth of hardness from 0.8-1.6 mm is attained in three to four hours. The gears are then allowed to cool slowly within the box and then removed. The second stage consists of reheating the gears (so obtained) to about 900°C and then quenching in oil so that its structure is refined, brittleness removed, and the core becomes soft and tough. The metal is then reheated to about 700°C and quenched in water so that the outer surface of gear, which had been rendered soft during the preceding operation, is again hardened.
The difference between liquid carburizing and cyaniding is that carburizing offers advantages because it supplies case hardening for the exterior surfaces of low carbon steel and iron alloys. Metal parts which undergo heat treatment and carburizing tend to resist abrasions more effectively.
Carbonitriding is similar to cyaniding except that the heat treatment is conducted in a gaseous atmosphere of ammonia and hydrocarbons instead of sodium cyanide. If the part is to be quenched, it is heated to 775 to 885°C (1,427 to 1,625°F). If the processing plan for this particular part does not include quenching, then the part is heated to only 649 to 788°C (1,200 to 1,450°F).
The Difference Between Liquid Carburizing and Cyaniding or Pack Cyanide
Cyaniding and carbonitriding are two forms of case hardening processes that are useful in getting a hard surface on metal. The key difference between cyaniding and carbonitriding is that cyaniding uses sodium cyanide liquid, whereas the carbonitriding process, as noted above, uses a gaseous atmosphere consisting of ammonia and hydrocarbons. Moreover, cyaniding involves temperatures around 871 to 954°C. In carbonitriding, if the part is to be quenched, the temperature of the bath is maintained between 445 to 885°C. If the part is not going to be quenched, then the metal surface temperature would be maintained between 649 to 788°C.
Another use of pack cyanide or cyaniding is in metal finishing, widely used in electroplating industries in order to assure the high quality of the finished products. Consequently, it is a common contaminant in different industrial effluents including metal processing, gold mining, and plastics, which generally co-exist with other toxic substances such as heavy metals.
The disadvantages of cyaniding are its high cost and the toxicity of the cyanide salts, the latter necessitating the adoption of special measures to protect workers and the environment.
To briefly summarize the differences between a carburizing heat treatment and nitriding:
• Carburizing is a heat-treatment process that diffuses carbon into the surface of a metal to create a hardened surface, whereas nitriding is a heat-treating process that diffuses nitrogen into the surface of a metal to create a hardened surface.
• Carburizing uses a carbonaceous environment; nitriding uses nitrogen instead of carbon.
• Carburizing is done at very high temperatures; nitriding can be done at low temperatures.
• In carburizing, carbon is diffused onto the surface of the metal alloy. In nitriding, nitrogen is diffused onto the surface of the metal alloy.
Carburizing and nitriding are two types of surface hardening processes that are used to make a steel surface hard while the core remains soft. Reiterating, the main difference between carburizing and nitriding is that in carburizing, carbon is diffused to the steel surface whereas, in nitriding process, nitrogen is diffused to the steel surface.
Complementary Processes
Ferritic nitrocarburizing (FNC). This is a surface engineering process for the heat treatment of steels and cast irons performed within a temperature band 550 to 740°C (1,020 to 1,350 F). All heat treatment is done in a gaseous environment, rich in nitrogen. The objective is to develop an iron nitride surface layer of approximately 5 to 50 (mm). This is in turn supported by a nitrogen-rich diffusion zone in the underlying material. The ferritic nitrocarburizing diffuses mostly nitrogen and some carbon into the case of a workpiece below the critical temperature, approximately 650°C (1,202°F), which is under the critical temperature of the microstructure of the material, which does not convert to an austenitic phase but stays in the ferritic phase, which is why it is called ferritic nitro-carburization.
The FNC process result provides for a high surface hardness of the compound layer with a surface microporosity, which enhances lubricant retainment (which in turn delivers excellent wear-resistant function). The specifics of the developed surface layer provide for high resistance to indentation of plastic deformation; thus, it provides excellent resistance to surface distress and cumulative damage.
The FNC process is done at temperatures below that wherein internal and surface stresses are relieved during heat treatment. Thus, this process does not cause distortion common to the amount caused by processes such as case carburizing. To be clear, FNC does not generate a thick (relative) layer as does case carburizing, which affords parts treated via case carburizing the ability to sustain more surface distress and the follow-on cumulative damage and degradation of the contact surface (e.g. micro- progressing to pitting and subsequent spalling).
Nitriding: This is another case-hardening process wherein nitrogen is used to saturate the surface of a part and hold it for prolonged periods of time, generally at temperature from 480 to 650°C in an atmosphere of ammonia gas (NH3). The nitrogen from the ammonia gas enters into / onto the surface of the steel and forms nitrides that impart extreme hardness to surface. Nitriding is a case-hardening process in which nitrogen instead of carbon is added to the outer skin of the steel. This is a heat-treating process that diffuses nitrogen onto (with only a slight infusion layer into the part, on the order of a few ten-thousands of an inch) the surface of a metal to create a hardened surface. The nitriding process uses nitrogen and heat. This is commonly used for fuel injection pumps. In this method, nitrogen is diffused to the steel surface instead of carbon. Nitriding can be done at lower temperatures than carburizing. This process is used for those alloys which are susceptible to the formation of chemical nitrides.
The part to be nitrided is placed in a container (usually made of high nickel chromium steel) and with inlet and outlet tubes through which ammonia gas is circulated. Ammonia gas is used as a nitrogen-producing component. The alloy steel containing Cr, Ni, Al, Mo, V and other nitrile alloys are widely used for this process. Plain carbon steels are seldom nitirided. Nitride-forming elements must be present for this method to work; these elements include chromium, molybdenum, and aluminum. The advantage of this process is that it causes little distortion, so the part can be case-hardened after being quenched, tempered, and machined. No quenching is typically done after nitriding.
Nitriding is a better case-hardening process than carburizing or cyaniding, because in this method, nitrogen is diffused to the steel surface instead of carbon. Nitriding can be done at lower temperatures than carburizing. The diffusion of nitrogen gas normally occurs at low temperatures, and hardening occurs without quenching. Only the surface is hardened, the core remains at the same basic properties and hardness.
References
- “Cyaniding and Nitriding,” by Faizan Ali.
- “Computerized Heat Treatment Furnace,” by S. Zillayali.
- “Carburizing Process and Carburizing Steels,” by A. Satyendra.
- “The Effects of Pack Carburizing Using Charcoal on Properties of Mild Steel,” by S. Supriyono.
- “Pack Carburization of Mild Steel, using Pulverized Bone as Carburizer: Optimizing Process Parameters,” by F. Aramide and S. Ibitoye.