
First in a series » Among the many specifications applied to finished heat-treated goods, the cleanliness of those goods is increasingly important in appearance, performance, and customer satisfaction. In this series of articles, I’ll break down the why and how of cleaning for heat treatment.
In this short column, we will discuss the importance of cleaning in heat-treating operations.
Introduction
The demands on heat-treated goods are increasing. Not only do they have to meet property and straightness requirements, but they also must meet increasingly stringent appearance specifications. Cleaning is the removal of dirt and soil from a surface. A cleaner is a formulated chemical system or product, dispersed in water, which removes unwanted dirt, soil, grease, and other similar matter from a surface.
Cleaners are used in nearly all industrial plants where metal-working operations are found. Almost all soil and dirt needs to be removed to some extent. Thus, if a plant uses coolants, drawing compounds, rust preventatives, or heat-treating oils, then the plant will likely have a strong requirement for cleaning.
Cleanliness is a relative term. The total process will dictate the degree of cleanliness. For example, a part is being cleaned during various states of manufacture to remove chips or machinery fluids. The cleaner will leave behind a residual rust protective film. Although there is residual film on the part, it is considered clean. In the case of electroplating, blackening, or enameling, the part must be chemically clean. This is often referred to by the term “water break free.” After cleaning, if a part is rinsed in clear water, the water should run down the part in a continuous, unbroken film. This indicates a water-break free part. If the film is interrupted, this indicates some soil is remaining on the surface. Cleanliness should be determined by the customer. During a cleaner trial, it is good policy to allow the customer to make the initial comments concerning cleanliness. A part which may appear to be dirty to an operator could be quite acceptable to the final customer.
Factors affecting industrial cleaning
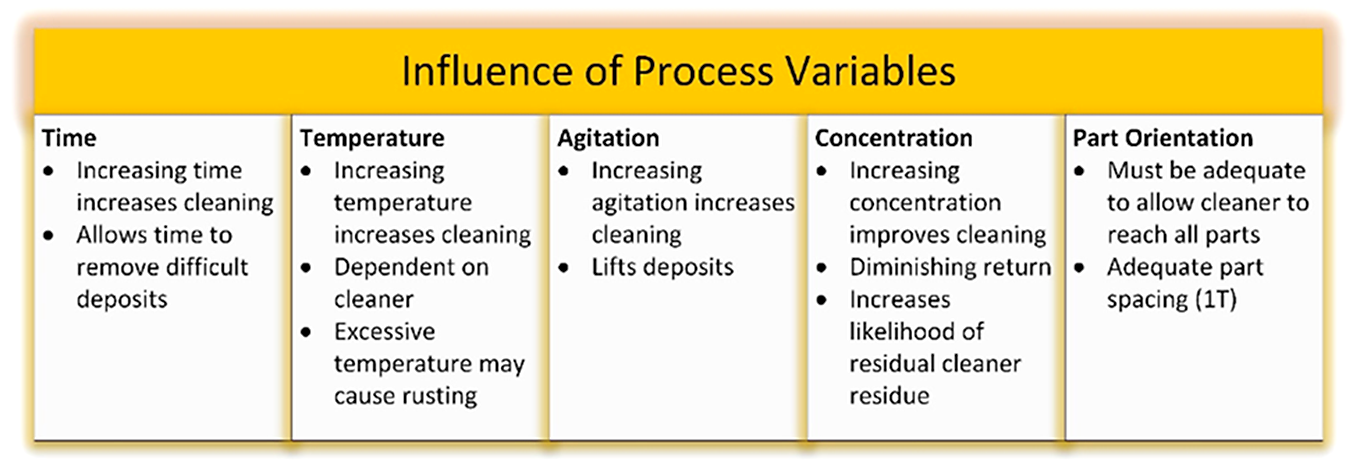
With a few exceptions, there are basic variables that apply to all types of cleaning. These are concentration of the cleaner, time required for cleaning, temperature, agitation, and water quality (hardness).
Concentration. The concentration necessary for good cleaning is geared to the method of agitation. Thus, disregarding any foam problems, it is possible to get similar cleaning from the same alkaline cleaner at 8 to 12 oz./gal. by soaking, 1/2 to 1oz./gal. by spraying, 1/10 to 1/2 oz./gal. by steam cleaning, or 2 to 4 oz./gal. in a high-pressure water spray at room temperature.
Time Required for Cleaning. This is determined by the conditions of temperature, soil, concentration, agitation, etc. Keep in mind that it does take some time for the detergency process to take place. In a static bath, cleaning may take 5 to 15 minutes; in a high-pressure spray of quality detergent, it may take a few seconds. It is expensive in equipment, labor, and chemicals to reduce cleaning time, and there are situations in which the expense is not justified.
The time available for cleaning is very closely related to the economics of the cleaning operation not only from a direct production viewpoint, but also based on time lost in recleaning, rejects, or customer complaints when inadequate cleaning results from an unsuccessful balance of cleaning factors.
Temperature. The effect of temperature depends on the type of soil and cleaner. A first consideration is that enough temperature to melt a soil that can be melted, such as a fat, grease, or wax, makes a tremendous difference in the rate of cleaning. For most oils, increase of temperature reduces the viscosity of the oil, makes it more mobile, and therefore more easily removed. There is a well-established principle that the rate of chemical reaction is doubled for every 10°C or 18°F increase in temperature. If cleaning is by chemical reaction between a fatty oil and alkali, or if a paint or foodstuff is being decomposed by chemical action, or if a rust or scale remover is acting chemically, this same relationship occurs. Most industrial cleaning is carried out at temperatures of 140-180°F.
Agitation. Variation in agitation represents the greatest difference in the methods of cleaning that I have discussed so far. An important factor is that it costs money in equipment or labor, or both, to provide high levels of agitation. Equipment design has the objective of getting the amount of agitation necessary to remove the soil as rapidly as is needed at the lowest cost; the last two factors often require a compromise. Agitation should be used with the idea of moving the soil. Circulation or stirring the cleaner is far less effective but is sometimes useful. High-pressure spraying of cleaner is most effective. A great deal of attention has been paid to obtaining good agitation to improve cleaning. This may vary from the unsophisticated scrub brush to mild-pressure sprays (5 – 40 psi), to high-pressure sprays (300 – 1,000 psi), to the current density electro-cleaning, to high-intensity ultrasonic cleaning, etc. Bubbling air through the cleaner or stirring with a mixer, or sloshing the work piece around in the cleaner are less effective methods of agitation. Major limitations of agitation are equipment expense, foaming, generation of irritating fog, toxic vapors, or flammable gases.
 Water Hardness. Water hardness depends upon the concentration of chemical salts dissolved in the water. These salts, in the presence of heat or alkalinity, may react to produce insoluble material which precipitates out of solution in the form of hard water scale. Unless this formation of hard water scale is prevented, several undesirable results are possible. The major undesirable result is the buildup of hard-water scale. This scale may be precipitated on the heating coils of wash tanks, reducing the efficiency and wasting energy and money.
Water Hardness. Water hardness depends upon the concentration of chemical salts dissolved in the water. These salts, in the presence of heat or alkalinity, may react to produce insoluble material which precipitates out of solution in the form of hard water scale. Unless this formation of hard water scale is prevented, several undesirable results are possible. The major undesirable result is the buildup of hard-water scale. This scale may be precipitated on the heating coils of wash tanks, reducing the efficiency and wasting energy and money.
Scale may build up in spray nozzles and on belt conveyors, impairing cleaning efficiency. The effectiveness of the spray is reduced, and scale may block the solution from reaching and cleaning the objects on the belt. Scale may also build up in the rinse tanks in extremely hard water situations due to alkaline carryover of cleaner into the rinse tank. Hard water salts regularly react with fatty acid soaps to yield insoluble residues on parts being cleaned. The harder the water is, the greater the susceptibility to rusting parts and the washer. Hard water is not conducive to good cleaning.
A summary of the effects of process variables on cleaning is shown in Figure 1.
Conclusion
In this column, I have discussed the effect of various process variables on cleaning. In the next column, I will discuss types of soils on heat-treated parts.
Should you have any questions on this article, or suggestions for further columns, please contact the editor or myself.























