
Cold treatment is a sub-zero thermal treatment process primarily used to reduce the retained austenite content of alloy and high carbon steels. Cold treatment covers the approximate temperature range of 0°C to 80°C, below which is considered cryogenic or deep cryogenic treatment [1]. Although cold treatment has been used to improve the performance of steel for centuries [2], it continues to be a topic of discussion in many industries [3-5]. As a result, the following discussion and analysis is intended to provide insights into the cold-treatment processes as well as provide context to identify key factors to be considered when establishing a cold-treatment process.
Cold-Treatment Fundamentals
Many steels used in a heat-treated condition are heated to form a significant fraction of austenite and then cooled to form a variety of microstructures depending on the required properties for the application. Martensite is formed during rapid cooling or at a rate sufficiently fast to avoid formation of ferrite, pearlite, and bainite. The temperature at which martensite forms is designated in literature as the martensite start (Ms) temperature. The Ms represents the thermodynamic driving force necessary to initiate the austenite-to-martensite shear transformation [6]. Chemical composition of the steel affects the Ms temperature. Carbon suppresses the Ms temperature significantly due to its ability to solid-solution strengthen austenite. The resultant is that higher carbon contents require higher shear stresses and therefore greater undercooling to initiate the martensite transformation. Empirical equations for the Ms temperature as a function of chemical composition have been developed for a variety of steel classifications; however, the Andrews linear equation
![]() from 1965 is still one of the simplest and most widely used. In the Andrews equation, Ms has units of degrees Celsius and alloying additions of carbon (C), manganese (Mn), chromium (Cr), nickel (Ni), and molybdenum (Mo) are in weight percent (wt%) [6]. The Andrews equation was determined using steels of the following composition range, in wt%: <0.6 C, 0.6-4.9 Mn, <5 Cr, <5 Ni, <5.4 Mo. Although high-carbon steels are excluded from this chemical composition range, use of the Andrews equation as a first approximation is common. Since the Ms reflects a thermodynamic value, the extent of the athermal martensite transformation can be quantified as a function of undercooling below the Ms. The Koistinen-Marburger equation
from 1965 is still one of the simplest and most widely used. In the Andrews equation, Ms has units of degrees Celsius and alloying additions of carbon (C), manganese (Mn), chromium (Cr), nickel (Ni), and molybdenum (Mo) are in weight percent (wt%) [6]. The Andrews equation was determined using steels of the following composition range, in wt%: <0.6 C, 0.6-4.9 Mn, <5 Cr, <5 Ni, <5.4 Mo. Although high-carbon steels are excluded from this chemical composition range, use of the Andrews equation as a first approximation is common. Since the Ms reflects a thermodynamic value, the extent of the athermal martensite transformation can be quantified as a function of undercooling below the Ms. The Koistinen-Marburger equation
![]() relates the martensite volume fraction, f, to the undercooling below Ms, Δ T, in degrees Celsius which is equivalent to cold-treatment temperature in the present context. It is important to emphasize the Koistinen-Marburger equation is independent of time.
relates the martensite volume fraction, f, to the undercooling below Ms, Δ T, in degrees Celsius which is equivalent to cold-treatment temperature in the present context. It is important to emphasize the Koistinen-Marburger equation is independent of time.
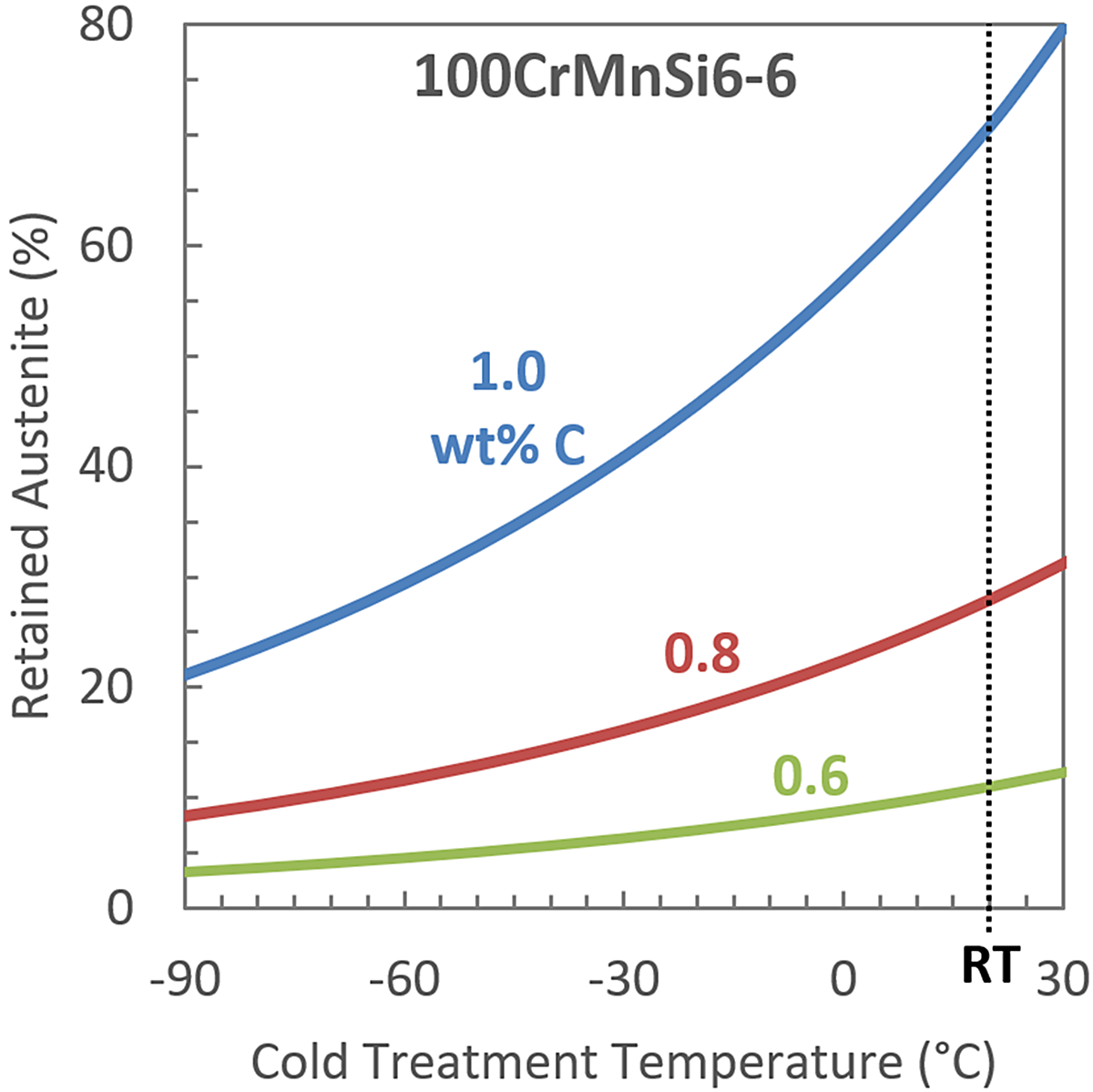
Figure 1 shows the plot that can be made by combining the Andrews and Koistinen-Marburger equations. Retained austenite (RA) as a function of cold-treatment temperature was calculated using the two equations along with the nominal chemical composition of 100CrMnSi6-6 [7]. Lines of constant C content for 0.6, 0.8, and 1.0 wt% C were shown to demonstrate the significant influence C content has on the fraction of retained austenite for a given alloy composition and cold-treatment temperature. At room temperature (RT, +20°C) a 1 wt% C version of 100CrMnSi6-6 is estimated to have 70 percent RA while a 0.6 wt% C version is estimated to only have 10 percent RA. In high-carbon steels, the amount of carbon in the austenite depends primarily on the temperature the steel is austenitized. Figure 2 shows published data relating the Ms temperature to the austenitizing temperature of 52100. The Ms temperature decreases as austenitizing temperature increases, approaching a constant Ms temperature of approximately 140°C at the highest austenitizing temperatures evaluated. In the range of austenitizing temperatures most relevant to industrial heat treatment of 52100, less than 900°C, the Ms temperature is very sensitive to austenitizing temperature and therefore requires strict process control.
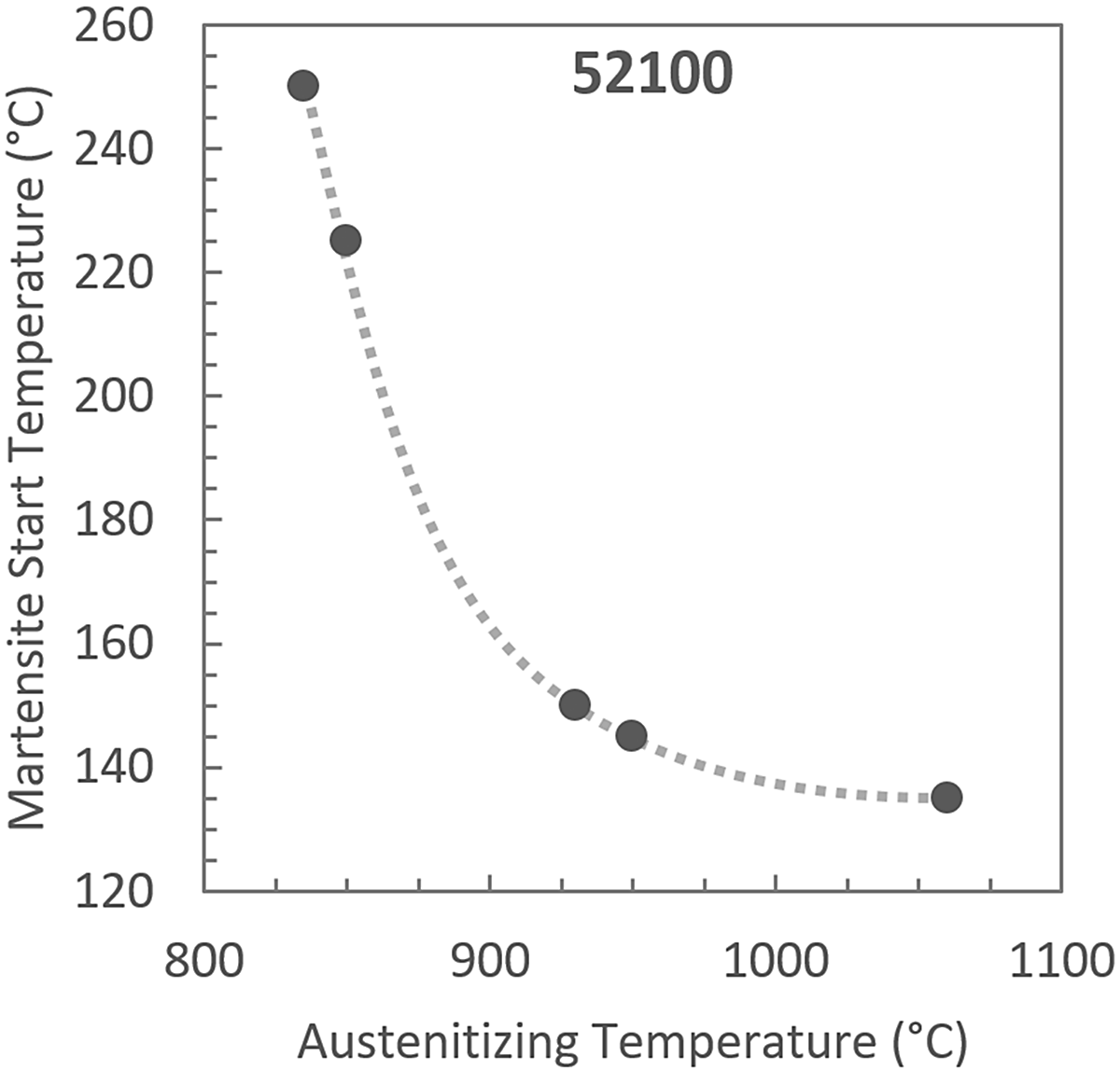
Figure 3 shows four representative micrographs of 100CrMnSi6-6 quenched to room temperature as well as cold treated at three different temperatures. A single specimen was austenitized in a protective atmosphere furnace, oil quenched to room temperature, cut, mounted, polished, lightly etched, and cold treated at –20, –60, and –80°C to visually demonstrate the evolution of retained austenite fraction during cold treatment. The sample was austenitized at a sufficiently high temperature to allow nearly all carbon to be dissolved in the austenite before being quenched to room temperature. White areas are retained austenite, brown/tan areas are martensite, and the gray globular feature near the center of each image is a manganese sulfide (MnS) and was used as a fiducial marker to ensure image position was not lost between each cold treatment.
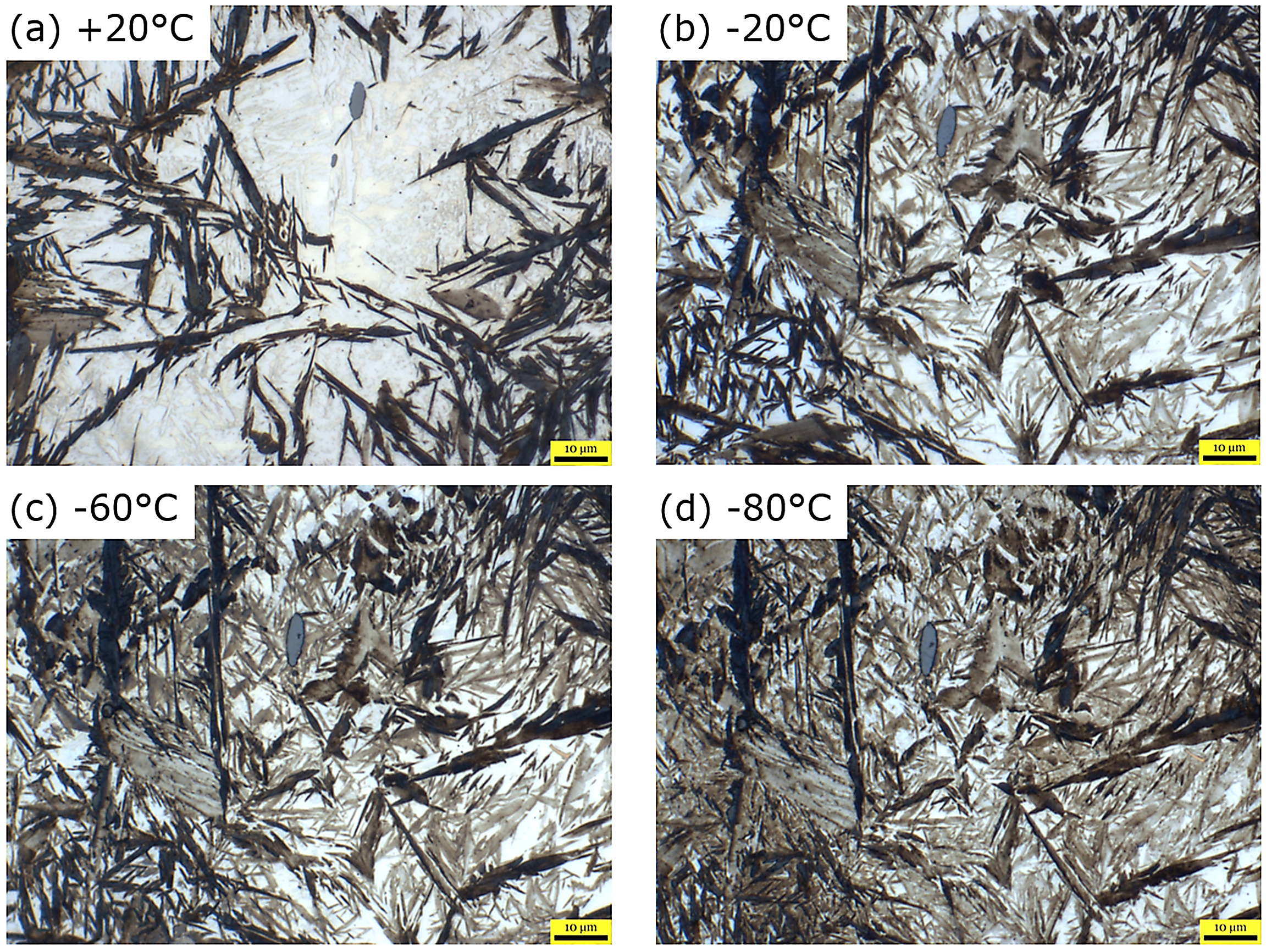
Figure 4 shows the hardness and visual retained austenite data collected from the specimen shown in Figure 3. Each data point represents the sample mean ±1σusing five HRC measurements for hardness and five 200x magnification fields for visual retained austenite. The room temperature condition (+20°C) exhibited approximately 53 HRC at 70 percent RA while the -80°C cold-treated condition exhibited approximately 58.5 HRC at 25 percent RA.
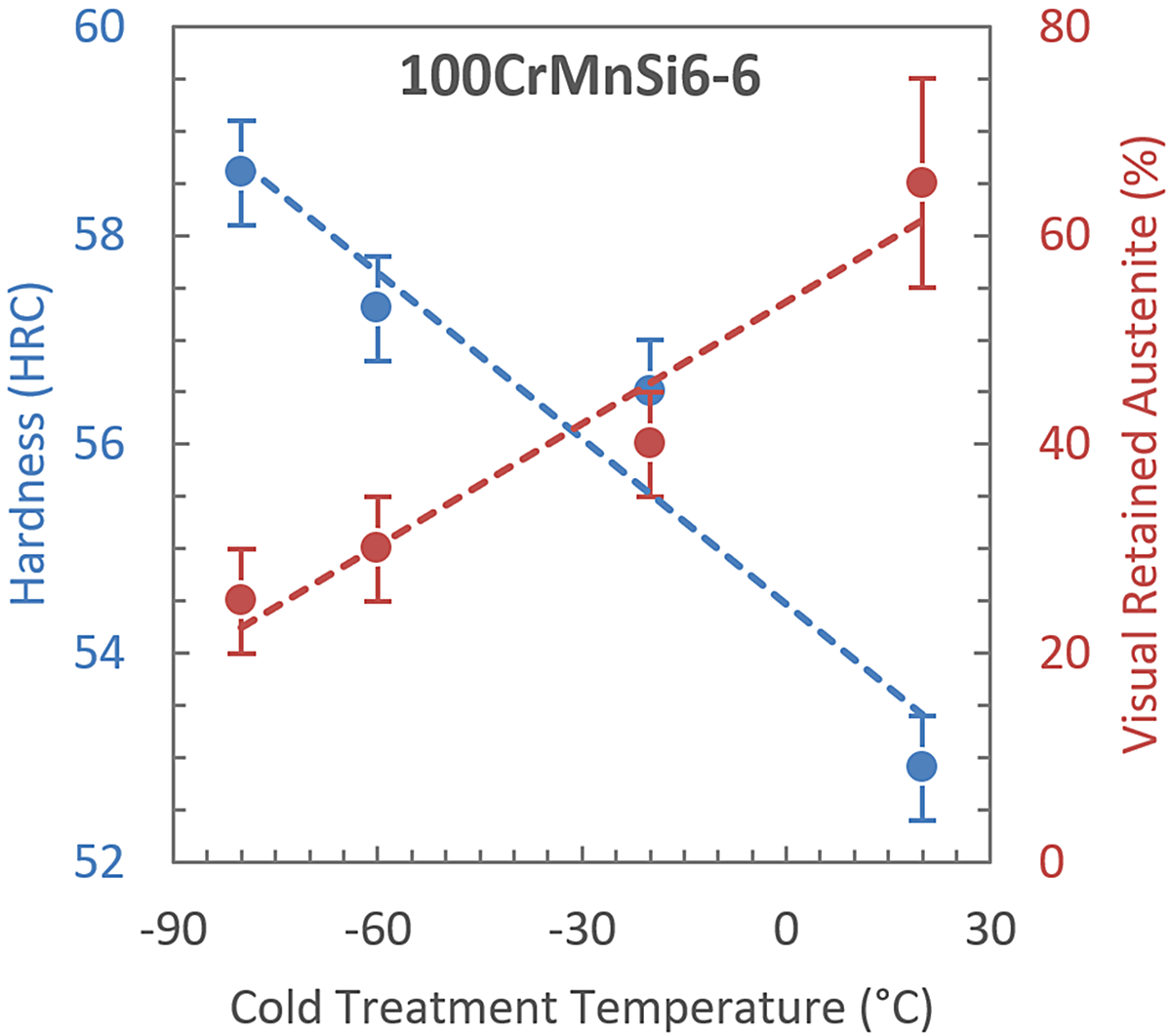
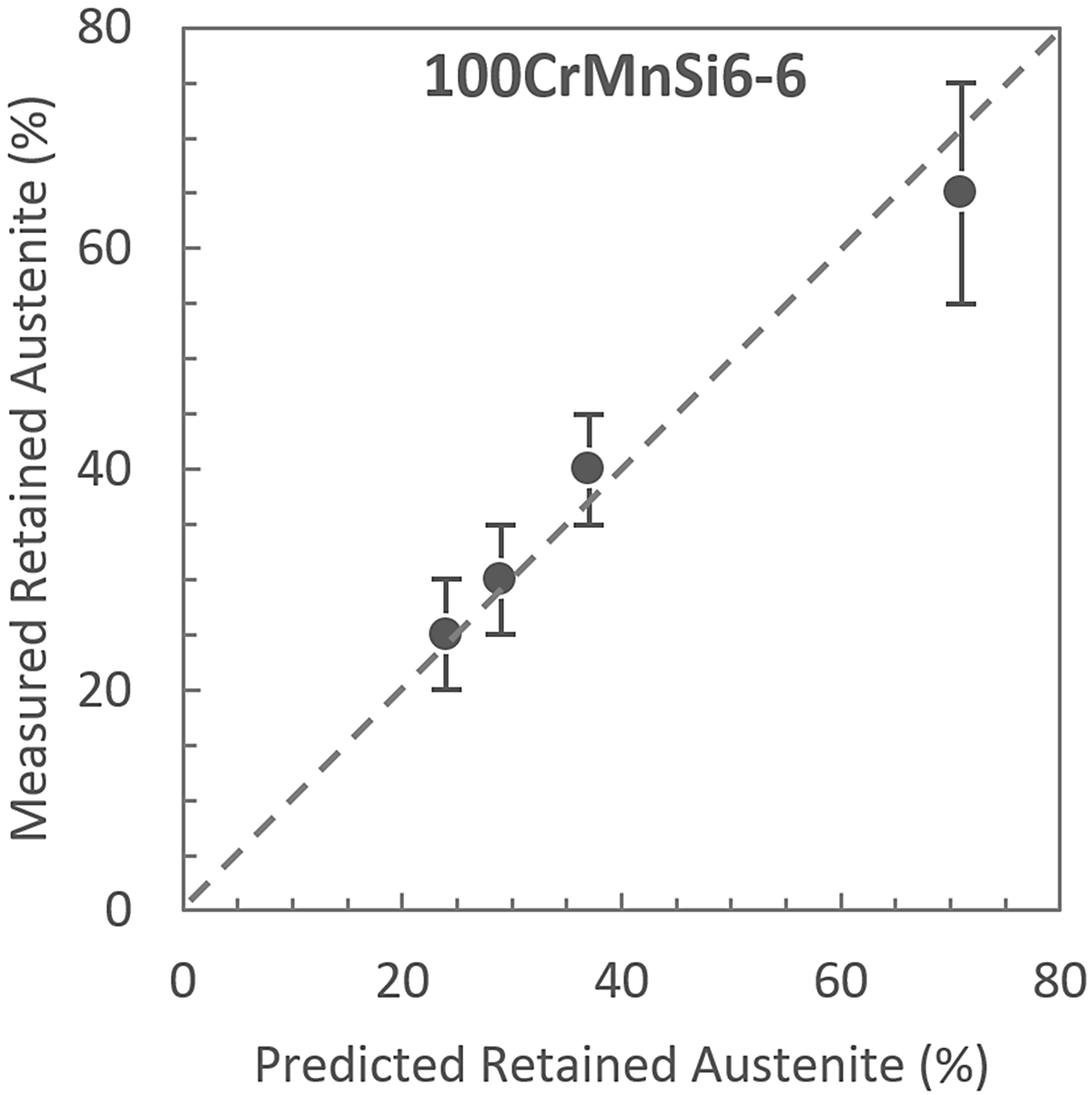
Figure 5 compares the measured values shown in Figure 4 and the calculated values obtained using both the Andrews and Koistinen-Marburger equations shown in Figure 1. Although the data presented in this analysis is limited, it is in reasonably good agreement with the values calculated from literature.
Understanding the Process
The above-mentioned experimental data and analysis were presented as an exercise in linking established relationships from literature to results from a cold-treatment process. Although not every experiment will be in good agreement with literature, it allows for a basis of identifying and understanding the potential factors contributing to any misalignment. From the analysis above, austenite-carbon content and cold-treatment temperature can be considered critical factors to the control of a cold-treatment process. The latter of the two factors being the least significant.
Controlling austenite carbon content
The coefficient for C in the Andrews equation is an order of magnitude higher than any other alloying element in the equation. This clearly indicates the relative importance of controlling carbon content to ensure a consistent Ms is achieved. Methods for controlling austenite carbon content are provided below.
Starting microstructure: Consistency of austenitization is important to ensure a cold-treatment process provides consistent results. Spheroidized microstructures can be particularly challenging due to the potential for large variations in carbide size distribution.
Austenitizing temperature: Generally considered a coarse process adjustment. Figure 2 provided a sense as to the sensitivity of this process parameter in controlling austenite-carbon content.
Austenitizing time: Generally considered a fine process adjustment. Adjustment of the temperature should be first and then the time can be adjusted to ensure the process can accommodate small process anomalies.
Controlling cold-treatment temperature
Figure 1 showed controlling the cold-treatment temperature increases in importance as C content increases and higher temperature cold treatments can leave an appreciable amount of RA in the microstructure. The Koistinen-Marburger equation also showed the fraction of RA transformed is independent of time. The steel simply needs to uniformly get to temperature to achieve desired results.
Summary
Cold treatment of steels was discussed in the context of two equations from literature, the Andrews equation and the Koistinen-Marburger equation. The Andrews equation relates chemical composition of a steel to its Ms temperature, the thermodynamic driving force for martensite formation. The Koistinen-Marburger equation relates the fraction of martensite transformed to the amount of uncooling a steel has below its Ms temperature. Experimental data was in good agreement with the equations from literature. Analysis of the equations indicates austenite-carbon content is the primary controlling factor in a cold-treatment process while the cold-treatment temperature itself is secondary.
References
- ASM Handbook Vol. 4A: Steel Heat Treating Fundamentals and Processes, ASM International, Materials Park, OH, 2014.
- D.S. MacKenzie and G. Graham, “Beer, blood and urine – mythological quenchants of ancient blacksmiths,” in: Proceedings of the 23rd IFHTSE Congress, ASM International, Ohio, USA, 2016, pp. 101–109.
- A. Bensliey, et. al., “Cryogenic Treatment of Gear Steel.” Gear Solutions Magazine, Oct. 2011, pp. 36-50.
- G. Moroz and M.J. Stempo, “Sub-Zero Treatment of Steel Alloys Helps Satisfy Demand for Performance.” Process Cooling Magazine, Mar. 2015.
- A. Malas, “Extending Performance: Can sub-zero treatments give robotic gears a hand?” Thermal Processing Magazine, Sept. 2017.
- [G. Krauss, Steels – Processing, Structure, and Performance, 2nd ed., ASM International, Materials Park, OH, 2015.
- ASTM International. ASTM A485-17 Standard Specification for High Hardenability Antifriction Bearing Steel. West Conshohocken, PA; ASTM International, 2017.
- P. M. Unterweiser, H. E. Boyer, and J. J. Kubbs, Heat Treaters Guide, Metals Park, OH: American Society for Metals, 1982.



























